| Citation: | Wenqiang Wu, Kaihang Yue, Kang Zhang, Jingcheng Xu, Xia Bao Yu, Yan Ya, Xianying Wang. Ionic liquid induced controllable synthesis of nickel-hydroxide-encapsulated NiFe layered double hydroxide for efficient oxygen evolution[J]. Energy Lab, 2023, 1(3): 220020. doi: 10.54227/elab.20220020 |
Ionic liquid induced controllable synthesis of nickel-hydroxide-encapsulated NiFe layered double hydroxide for efficient oxygen evolution
-
Abstract
The efficiency of electrochemical water splitting is severely restricted by the slow oxygen evolution reaction (OER) on the anode. Therefore, the design and synthesis of high-performance electrocatalysts for anodic oxygen evolution is crucial for the industrialization of hydrogen production by electrolysis of water. Herein, an efficient core-shell Ni(OH)2@NiFe LDH electrocatalyst was designed with the assistant of ionic liquid for OER. The ionic liquid delayed the crystallization ability of Ni2+ ions and then facilitated the formation of NiFe LDH coated Ni(OH)2 structure. The as-obtained core-shell Ni(OH)2@NiFe LDH exhibited outstanding OER electrocatalytic activity that only required overpotentials of 258 mV to deliver current densities of 100 mA cm−2, and a decent stability of at least 300 h under a large current density of 100 mA cm−2. This study provides a valuable reference for the structure design of NiFe LDH based catalyst.
-
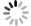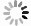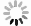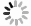
-
References
1. Y. Yan, J.-Y. Zhang, X.-R. Shi, Y. Zhu, C. Xia, S. Zaman, X. Hu, X. Wang and B. Y. Xia, ACS Nano, 2021, 15, 10286 2. Y. Zhu, K. Yue, C. Xia, S. Zaman, H. Yang, X. Wang, Y. Yan and B. Y. Xia, Nano-Micro Lett., 2021, 13, 137 3. Y. Yan, Y. Xu, B. Zhao, Y. Xu, Y. Gao, G. Chen, W. Wang and B. Y. Xia, J. Mater. Chem. A, 2020, 8, 5070 4. R. Gao and D. Yan, Nano Res., 2018, 11, 1883 5. Z. Guo, W. Ye, X. Fang, J. Wan, Y. Ye, Y. Dong, D. Cao and D. Yan, Inorg. Chem. Front., 2019, 6, 687 6. T. Binninger and M.-L. Doublet, Energy Environ. Sci., 2022, 15, 2519 7. J. Wang, C. Cheng, Q. Yuan, H. Yang, F. Meng, Q. Zhang, L. Gu, J. Cao, L. Li, S.-C. Haw, Q. Shao, L. Zhang, T. Cheng, F. Jiao and X. Huang, Chem, 2022, 8, 1673 8. R. Gao and D. Yan, Adv. Energy Mater., 2020, 10, 1900954 9. L. Zhang, J. Han, R. Wang, X. Qiu, and J. Ji, J. Chem. Eng. Data, 2007, 52, 1401 10. R. Chen, S.-F. Hung, D. Zhou, J. Gao, C. Yang, H. Tao, H. B. Yang, L. Zhang, L. Zhang, Q. Xiong, H. M. Chen and B. Liu, Adv. Mater., 2019, 31, 1903909 11. R. Gao, J. Zhu and D. Yan, Nanoscale, 2021, 13, 13593 12. L. Zhou, C. Zhang, Y. Zhang, Z. Li and M. Shao, Adv. Funct. Mater., 2021, 31, 2009743 13. Z. Cai, P. Wang, J. Zhang, A. Chen, J. Zhang, Y. Yan and X. Wang, Adv. Mater., 2022, 34, 2110696 14. L. Peng, N. Yang, Y. Yang, Q. Wang, X. Xie, D. Sun-Waterhouse, L. Shang, T. Zhang and G. I. N. Waterhouse, Angew. Chem. Int. Ed., 2021, 60, 24612 15. Z. Cai, X. Bu, P. Wang, W. Su, R. Wei, J. C. Ho, J. Yang and X. Wang, J. Mater. Chem. A, 2019, 7, 21722 16. X. Feng, Q. Jiao, W. Chen, Y. Dang, Z. Dai, S. L. Suib, J. Zhang, Y. Zhao, H. Li and C. Feng, Appl. Catal. B, 2021, 286, 119869 17. M. V. Fedorov and A. A. Kornyshev, Chem. Rev., 2014, 114, 2978 18. J. Sun, N. Guo, Z. Shao, K. Huang, Y. Li, F. He and Q. Wang, Adv. Energy Mater., 2018, 8, 1800980 19. B. Murugesan, N. Pandiyan, M. Arumugam, M. Veerasingam, J. Sonamuthu, A. R. Jeyaraman, S. Samayanan and S. Mahalingam, Carbon, 2019, 151, 53 20. J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780 21. L. Fan, L. Zhao, Y. Lv, T. Wang, Y. Tian, J. Fu and X. Liu, Inorg. Chem. Front., 2022, 9, 3679 22. J. Hong, T. T. Mengesha, S.-W. Hong, H.-K. Kim and Y.-H. Hwang, J. Korean Phys. Soc., 2020, 76, 264 23. C. Y. Xu and Y. X. Hua, Mater. Sci. Forum, 2011, 633, 1163 24. C. Li, Z. Zhang and R. Liu, Small, 2020, 16, 2003777 25. Y. Yan, G. Cheng, P. Wang, D. He and R. Chen, RSC Adv., 2014, 4, 49303 26. Y. Zhai, X. Ren, Y. Sun, D. Li, B. Wang and S. Liu, Appl. Catal. B, 2023, 323, 122091 27. J. Zhang, A. Wei, J. Liu, J. Zhu, Y. He and Z. Liu, J. Alloys Compd., 2022, 927, 166990 28. T. u. Haq, Y. Haik, I. Hussain, H. u. Rehman and T. A. Al-Ansari, ACS Appl. Mater. Interfaces, 2021, 13, 468 29. X. Ge, C. Gu, Z. Yin, X. Wang, J. Tu and J. Li, Nano Energy, 2016, 20, 185 30. J.-J. Lv, J. Zhao, H. Fang, L.-P. Jiang, L.-L. Li, J. Ma and J.-J. Zhu, Small, 2017, 13, 1700264 31. J. L. Gunjakar, B. Hou, A. I. Inamdar, S. M. Pawar, A. T. A. Ahmed, H. S. Chavan, J. Kim, S. Cho, S. Lee, Y. Jo, S.-J. Hwang, T. G. Kim, S. Cha, H. Kim and H. Im, Small, 2018, 14, 1703481 32. Y. Hou, M. R. Lohe, J. Zhang, S. Liu, X. Zhuang and X. Feng, Energy Environ. Sci., 2016, 9, 478 33. M.-F. Chiang and T.-M. Wu, Appl. Clay Sci., 2011, 51, 330 34. K. Yue, J. Liu, Y. Zhu, C. Xia, P. Wang, J. Zhang, Y. Kong, X. Wang, Y. Yan and B. Y. Xia, Energy Environ. Sci., 2021, 14, 6546 35. K. Yue, J. Liu, C. Xia, K. Zhan, P. Wang, X. Wang, Y. Yan and B. Y. Xia, Mater. Chem. Front., 2021, 5, 7191 36. Y. Zhu, L. Zhang, B. Zhao, H. Chen, X. Liu, R. Zhao, X. Wang, J. Liu, Y. Chen and M. Liu, Adv. Funct. Mater., 2019, 29, 1901783 37. J. Yang, X. Wang, B. Li, L. Ma, L. Shi, Y. Xiong and H. Xu, Adv. Funct. Mater., 2017, 27, 1606497 38. C. Hu and L. Dai, Adv. Mater., 2017, 29, 1604942 39. I.-K. Ahn, S.-Y. Lee, H. G. Kim, G.-B. Lee, J.-H. Lee, M. Kim and Y.-C. Joo, RSC Adv., 2021, 11, 8198 40. J. Liu, J. Wang, B. Zhang, Y. Ruan, H. Wan, X. Ji, K. Xu, D. Zha, L. Miao and J. Jiang, J. Mater. Chem. A, 2018, 6, 2067 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Schematic illustration of the formation and transform the process of Ni(OH)2@NiFe LDH.
-
Figure 2.
a-b SEM images, c TEM image, d HRTEM (inset shows the corresponding SAED pattern), and e HAADF-STEM, f line scanning profiles of Ni, Fe, O, N, B, and F recorded along the line shown in e, and g-l element mapping of the Ni(OH)2@NiFe LDH.
-
Figure 3.
a XRD patterns, b Raman spectra of the Ni(OH)2@NiFe LDH and NiFe LDH. c FT-IR spectra of Ni(OH)2@NiFe LDH, NiFe LDH, and BMIMBF4. XPS spectra for Ni(OH)2@NiFe LDH and NiFe LDH d Ni 2p, e Fe 2p, f O 1s, g N 1s, h B 1s, i F 1s.
-
Figure 4.
a LSV curves, b Tafel slopes, c Nyquist plots (inset: equivalent circuit model), d Cdl values, and e The amount of H2 and O2 catalyzed by the Ni(OH)2@NiFe LDH || NiFe LDH/Ni NCs f Chronoamperometric curves for the OER. g Polarization curves of the AEMWE cell using Ni(OH)2@NiFe LDH || NiFe LDH/Ni NCs on Ni foam (inset: the AEMWE cell photograph). h The durability of the AEMWE cell at different current densities.



 DownLoad:
DownLoad: