| Citation: | Qingling Hong, Boqiang Miao, Tianjiao Wang, Fumin Li, Yu Chen. Intermetallic PtTe metallene for formic acid oxidation assisted electrocatalytic nitrate reduction[J]. Energy Lab, 2023, 1(2): 220022. doi: 10.54227/elab.20220022 |
Intermetallic PtTe metallene for formic acid oxidation assisted electrocatalytic nitrate reduction
-
Abstract
Development of highly efficient electrocatalysts for selective electroreduction of nitrate is of great significance. In this work, the ultrathin intermetallic platinum-tellurium metallene (PtTe-ML) with atomic thickness is synthesized by simple liquid-phase chemical reduction. The introduction of Te atoms can sharply weaken the catalytic activity of Pt for the hydrogen evolution reaction. And, PtTe-ML exhibits superior catalytic activity for the nitrate reduction reaction (NO3−-ERR) than Pt black. In 0.5 M H2SO4 solution, PtTe-ML achieves an effective ammonia (NH3) production rate of 2.32 mg h−1 mgcat−1 and a Faradic efficiency of 95.5% at −0.04 V potential for NO3−-ERR. Meanwhile, the entry of Te atom isolates the continuous Pt active site and increases the proportion of the direct dehydrogenation pathway of the formic acid oxidation reaction (FAOR). Therefore, PtTe-ML also exhibits excellent FAOR activity due to the optimization of FAOR pathway. Then, anodic FAOR with low anodic oxidation potential is used to replace the oxygen evolution reaction with slow kinetic, so that the total electrolytic voltage of conventional electrochemical NH3 production can be effectively reduced. Consequently, the bifunctional PtTe-ML electrocatalyst requires only 0.4 V total voltage for FAOR assisted NH3 electroproduction. This work demonstrates a reaction coupling strategy to significantly improve the utilization rate of electric energy in electrochemical synthesis.
-
-
References
1. T. Ren, K. Ren, M. Wang, M. Liu, Z. Wang, H. Wang, X. Li, L. Wang and Y. Xu, Chem. Eng. J., 2021, 426, 130759 2. J. Yu, B. Chang, W. Yu, X. Li, D. Wang, Z. Xu, X. Zhang, H. Liu and W. Zhou, Carbon Energy, 2022, 4, 237 3. P. H. van Langevelde, I. Katsounaros and M. T. M. Koper, Joule, 2021, 5, 290 4. J. Yao and J. Yan, Sci. China. Chem., 2020, 63, 1737 5. S. Mukherjee, D. A. Cullen, S. Karakalos, K. Liu, H. Zhang, S. Zhao, H. Xu, K. L. More, G. Wang and G. Wu, Nano Energy, 2018, 48, 217 6. Q. Yao, J. Chen, S. Xiao, Y. Zhang and X. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 30458 7. Y. Sun, W. Wu, L. Yu, S. Xu, Y. Zhang, L. Yu, B. Xia, S. Ding, M. Li, L. Jiang, J. Duan, J. Zhu and S. Chen, Carbon Energy, 2022, 1, 1 8. Y. Zeng, C. Priest, G. Wang and G. Wu, Small Methods, 2020, 4, 2000672 9. S. Garcia-Segura, M. Lanzarini-Lopes, K. Hristovski and P. Westerhoff, Appl. Catal. B Environ., 2018, 236, 546 10. Y. Yao, S. Zhu, H. Wang, H. Li and M. Shao, Angew. Chem. Int. Ed., 2020, 59, 10479 11. X. Yang, S. Sun, L. Meng, K. Li, S. Mukherjee, X. Chen, J. Lv, S. Liang, H.-Y. Zang, L.-K. Yan and G. Wu, Appl. Catal. B Environ., 2021, 285, 119794 12. G. A. Attard, J. Souza-Garcia, R. Martinez-Hincapie and J. M. Feliu, J. Catal., 2019, 378, 238 13. M. Duca, N. Sacre, A. Wang, S. Garbarino and D. Guay, Appl. Catal. B Environ., 2018, 221, 86 14. Z. Mumtarin, M. M. Rahman, H. M. Marwani and M. A. Hasnat, Electrochim. Acta, 2020, 346, 135994 15. R. Chauhan and V. C. Srivastava, Chem. Eng. J., 2020, 386, 122065 16. Z. X. Ge, T. J. Wang, Y. Ding, S. B. Yin, F. M. Li, P. Chen and Y. Chen, Adv. Energy Mater., 2022, 12, 2103916 17. J.-Y. Zhu, Q. Xue, Y.-Y. Xue, Y. Ding, F.-M. Li, P. Jin, P. Chen and Y. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 14064 18. J. Li, G. Zhan, J. Yang, F. Quan, C. Mao, Y. Liu, B. Wang, F. Lei, L. Li, A. W. M. Chan, L. Xu, Y. Shi, Y. Du, W. Hao, P. K. Wong, J. Wang, S.-X. Dou, L. Zhang and J. C. Yu, J. Am. Chem. Soc., 2020, 142, 7036 19. J. Liu, T. Cheng, L. Jiang, A. Kong and Y. Shan, ACS Appl. Mater. Interfaces, 2020, 12, 33186 20. S. Luo, W. Chen, Y. Cheng, X. Song, Q. Wu, L. Li, X. Wu, T. Wu, M. Li, Q. Yang, K. Deng and Z. Quan, Adv. Mater., 2019, 31, 1903683 21. T. Zhu, Q. Chen, P. Liao, W. Duan, S. Liang, Z. Yan and C. Feng, Small, 2020, 16, 2004526 22. J. Gao, B. Jiang, C. Ni, Y. Qi, Y. Zhang, N. Oturan and M. A. Oturan, Appl. Catal. B Environ., 2019, 254, 391 23. I. Katsounaros and G. Kyriacou, Electrochim. Acta, 2008, 53, 5477 24. M. Bat-Erdene, A. S. R. Bati, J. Qin, H. Zhao, Y. L. Zhong, J. G. Shapter and M. Batmunkh, Adv. Funct. Mater., 2022, 32, 2107280 25. H. Yang, F. He, J. Shen, Z. Chen, Y. Yao, L. He and Y. Yu, Energy Lab, 2022, 1, 220007 26. L. Zeng, W. Chen, Q. Zhang, S. Xu, W. Zhang, F. Lv, Q. Huang, S. Wang, K. Yin, M. Li, Y. Yang, L. Gu and S. Guo, ACS Catal., 2022, 12, 11391 27. H. Yu, T. Zhou, Z. Wang, Y. Xu, X. Li, L. Wang and H. Wang, Angew. Chem. Int. Ed. Engl., 2021, 60, 12027 28. P. Mirzaei, S. Bastide, A. Aghajani, J. Bourgon, E. Leroy, J. Zhang, Y. Snoussi, A. Bensghaier, O. Hamouma, M. M. Chehimi and C. Cachet-Vivier, Langmuir, 2019, 35, 14428 29. Y. Xu, K. Ren, T. Ren, M. Wang, M. Liu, Z. Wang, X. Li, L. Wang and H. Wang, Chem. Commun., 2021, 57, 7525 30. M. Armbrüster, K. Kovnir, M. Behrens, D. Teschner, Y. Grin and R. Schlögl, J. Am. Chem. Soc., 2010, 132, 14745 31. Y. S. Kang, D. Choi, J. Cho, H.-Y. Park, K.-S. Lee, M. Ahn, I. Jang, T. Park, H. C. Ham and S. J. Yoo, ACS Appl. Energy Mater., 2020, 3, 4226 32. J. Yu, A. F. Kolln, D. Jing, J. Oh, H. Liu, Z. Qi, L. Zhou, W. Li and W. Huang, ACS Appl. Mater. Interfaces, 2021, 13, 52073 33. F. Li, Q. Xue, G. Ma, S. Li, M. Hu, H. Yao, X. Wang and Y. Chen, J. Power Sources, 2020, 450, 227615 34. L. An, H. Yan, B. Li, J. Ma, H. Wei and D. Xia, Nano Energy, 2015, 15, 24 35. S. Liu, S. Yin, L. Cui, H. Yu, K. Deng, Z. Wang, Y. Xu, L. Wang and H. Wang, Energy Lab, 2022, 1, 220005 36. T.-J. Wang, H.-Y. Sun, Q. Xue, M.-J. Zhong, F.-M. Li, X. Tian, P. Chen, S.-B. Yin and Y. Chen, Science Bulletin, 2021, 66, 2079 37. L. Tao, M. Sun, Y. Zhou, M. Luo, F. Lv, M. Li, Q. Zhang, L. Gu, B. Huang and S. Guo, J. Am. Chem. Soc., 2022, 144, 10582 38. K. Yin, Y. Chao, F. Lv, L. Tao, W. Zhang, S. Lu, M. Li, Q. Zhang, L. Gu, H. Li and S. Guo, J. Am. Chem. Soc., 2021, 143, 10822 39. T.-J. Wang, Y.-C. Jiang, J.-W. He, F.-M. Li, Y. Ding, P. Chen and Y. Chen, Carbon Energy, 2022, 4, 283 40. T. Shen, S. Chen, C. Zhang, Y. Hu, E. Ma, Y. Yang, J. Hu and D. Wang, Adv. Funct. Mater., 2022, 32, 2107672 41. W. Liang, Y. Wang, L. Zhao, W. Guo, D. Li, W. Qin, H. Wu, Y. Sun and L. Jiang, Adv. Mater., 2021, 33, 2100713 42. H. Wang, W. Wang, Q. Mao, H. Yu, K. Deng, Y. Xu, X. Li, Z. Wang and L. Wang, Chem. Eng. J., 2022, 450, 137995 43. Q. Xue, X.-Y. Bai, Y. Zhao, Y.-N. Li, T.-J. Wang, H.-Y. Sun, F.-M. Li, P. Chen, P. Jin, S.-B. Yin and Y. Chen, J. Energy Chem., 2022, 65, 94 44. L. Bu, Q. Shao, Y. Pi, J. Yao, M. Luo, J. Lang, S. Hwang, H. Xin, B. Huang, J. Guo, D. Su, S. Guo and X. Huang, Chem, 2018, 4, 359 45. K. Kovnir, M. Armbrüs ter, D. Teschner, T. V. Venkov, L. Szentmiklósi, F. C. Jentoft, A. Knop-Gericke, Y. Grin and R. Schlögl, Surf. Sci., 2009, 603, 1784 46. K. Tonnis, Z. Nan, J. Fang, R. Pavlicek, E. S. DeCastro and A. P. Angelopoulos, ACS Appl. Energy Mater., 2020, 3, 7588 47. Z. Peng, H. You and H. Yang, Adv. Funct. Mater., 2010, 20, 3734 48. G.-T. Fu, B.-Y. Xia, R.-G. Ma, Y. Chen, Y.-W. Tang and J.-M. Lee, Nano Energy, 2015, 12, 824 49. J. Geng, Z. Zhu, X. Bai, F. Li and J. Chen, ACS Appl. Energy Mater., 2020, 3, 1010 50. S. H. Ahn, Y. Liu and T. P. Moffat, ACS Catal., 2015, 5, 2124 51. A. Ferre-Vilaplana, J. Victor Perales-Rondon, J. M. Feliu and E. Herrero, ACS Catal., 2015, 5, 645 52. Y. Xu, Y. Wen, T. Ren, H. Yu, K. Deng, Z. Wang, X. Li, L. Wang and H. Wang, Appl. Catal. B Environ., 2023, 320, 121981 53. Y. L. Zhao, Y. Liu, Z. J. Zhang, Z. K. Mo, C. Y. Wang and S. Y. Gao, Nano Energy, 2022, 97, 107124 54. Q. Liu, Q. Liu, L. Xie, Y. Ji, T. Li, B. Zhang, N. Li, B. Tang, Y. Liu, S. Gao, Y. Luo, L. Yu, Q. Kong and X. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 17312 55. M. Liu, Q. Mao, K. Shi, Z. Wang, Y. Xu, X. Li, L. Wang and H. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 13169 56. Y. Xu, M. Wang, K. Ren, T. Ren, M. Liu, Z. Wang, X. Li, L. Wang and H. Wang, J. Mater. Chem. A, 2021, 9, 16411 57. J. Lim, C.-Y. Liu, J. Park, Y.-H. Liu, T. P. Senftle, S. W. Lee and M. C. Hatzell, ACS Catal., 2021, 11, 7568 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
-
Supplementary Information
Supplemental_information-2023-0022-R1 
Information
Article Metrics
-
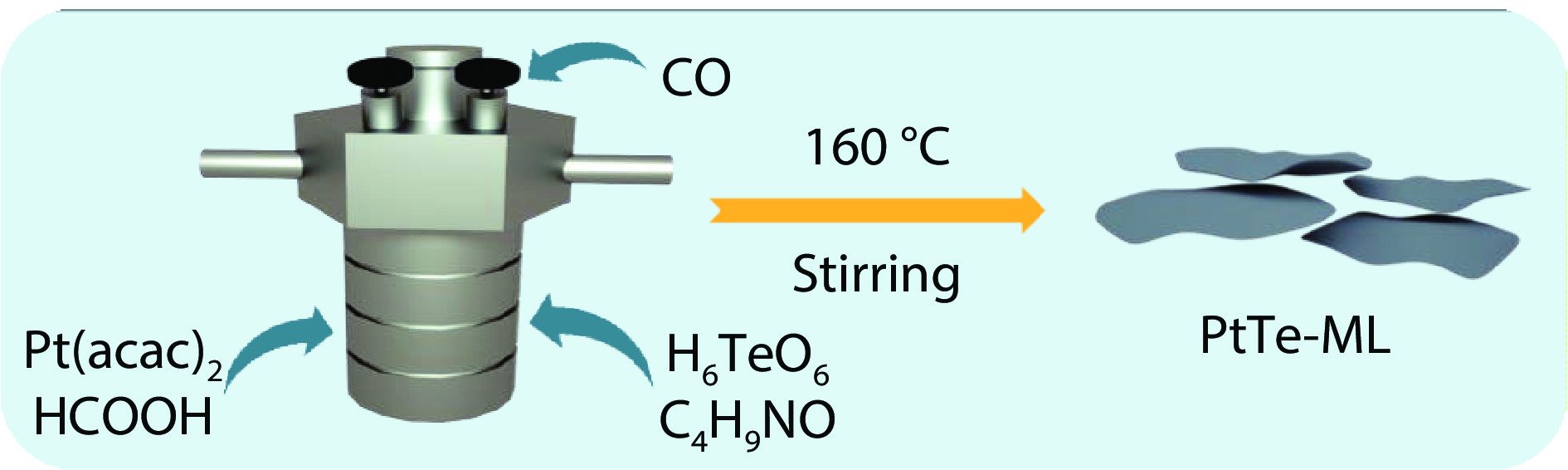
Figure 1.
Schematic illustration for PtTe-ML synthesis.
-
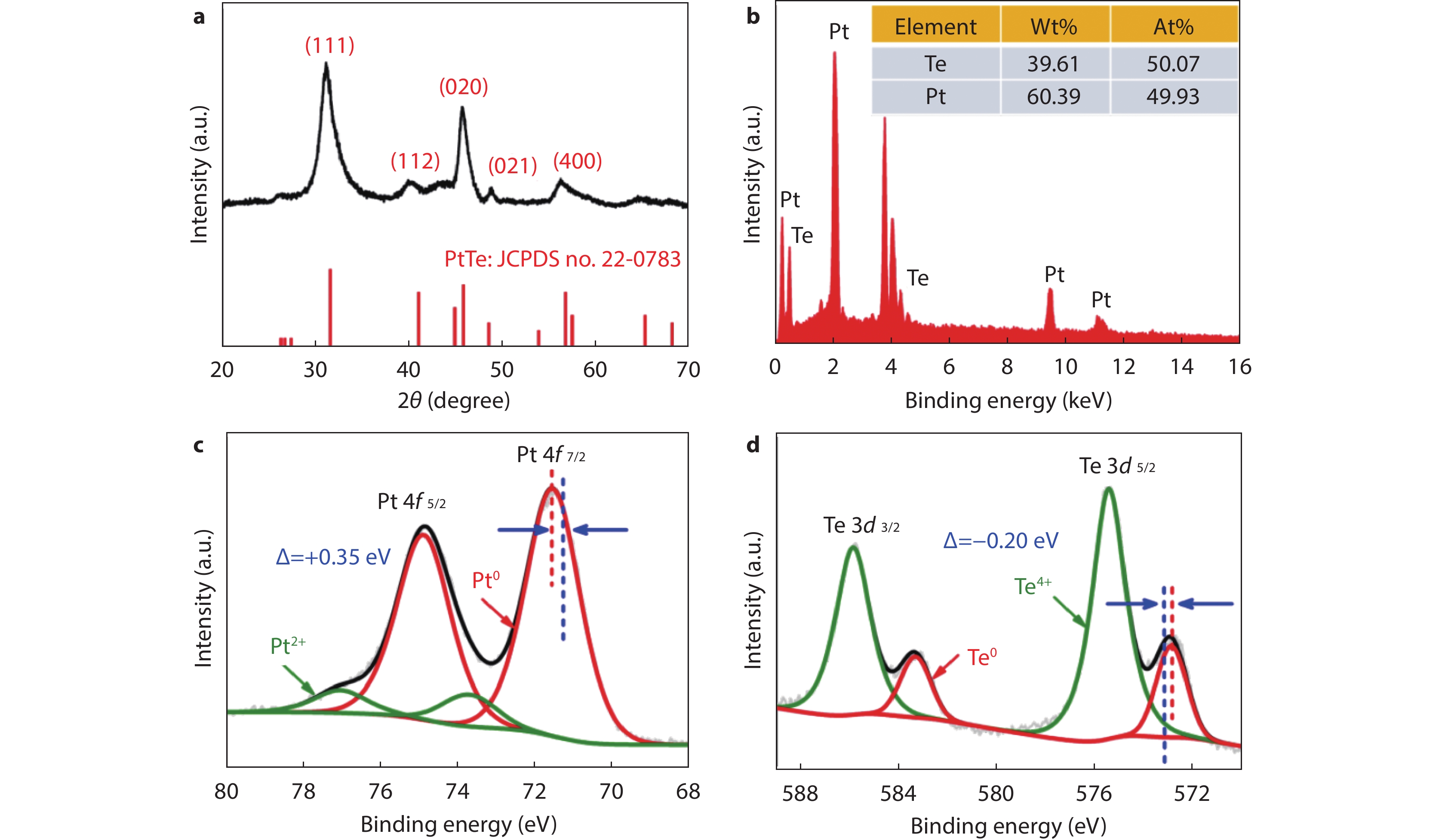
Figure 1.
a XRD pattern and b EDX spectrum of PtTe-ML. c Pt 4f and d Te 3d XPS spectra of PtTe-ML. The blue dotted lines in 1c and 1d represent the standard binding energy values of Pt and Te, respectively.
-
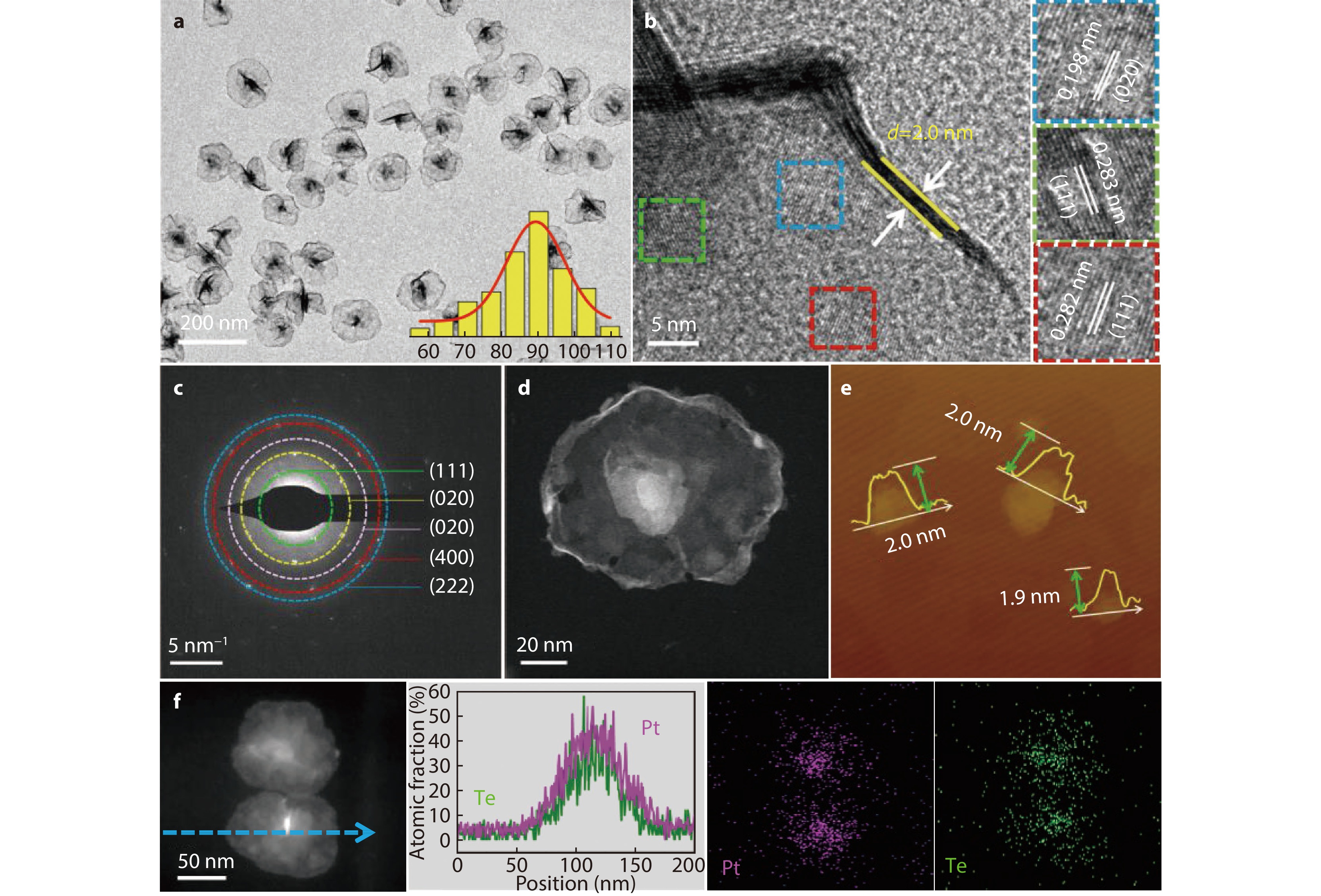
Figure 2.
a TEM image and size distribution histogram, b HRTEM image and magnified HRTEM image, c SAED pattern, d STEM image, and e AFM image of PtTe-ML. f STEM image and corresponding EDX mapping and line scanning of PtTe-ML.
-
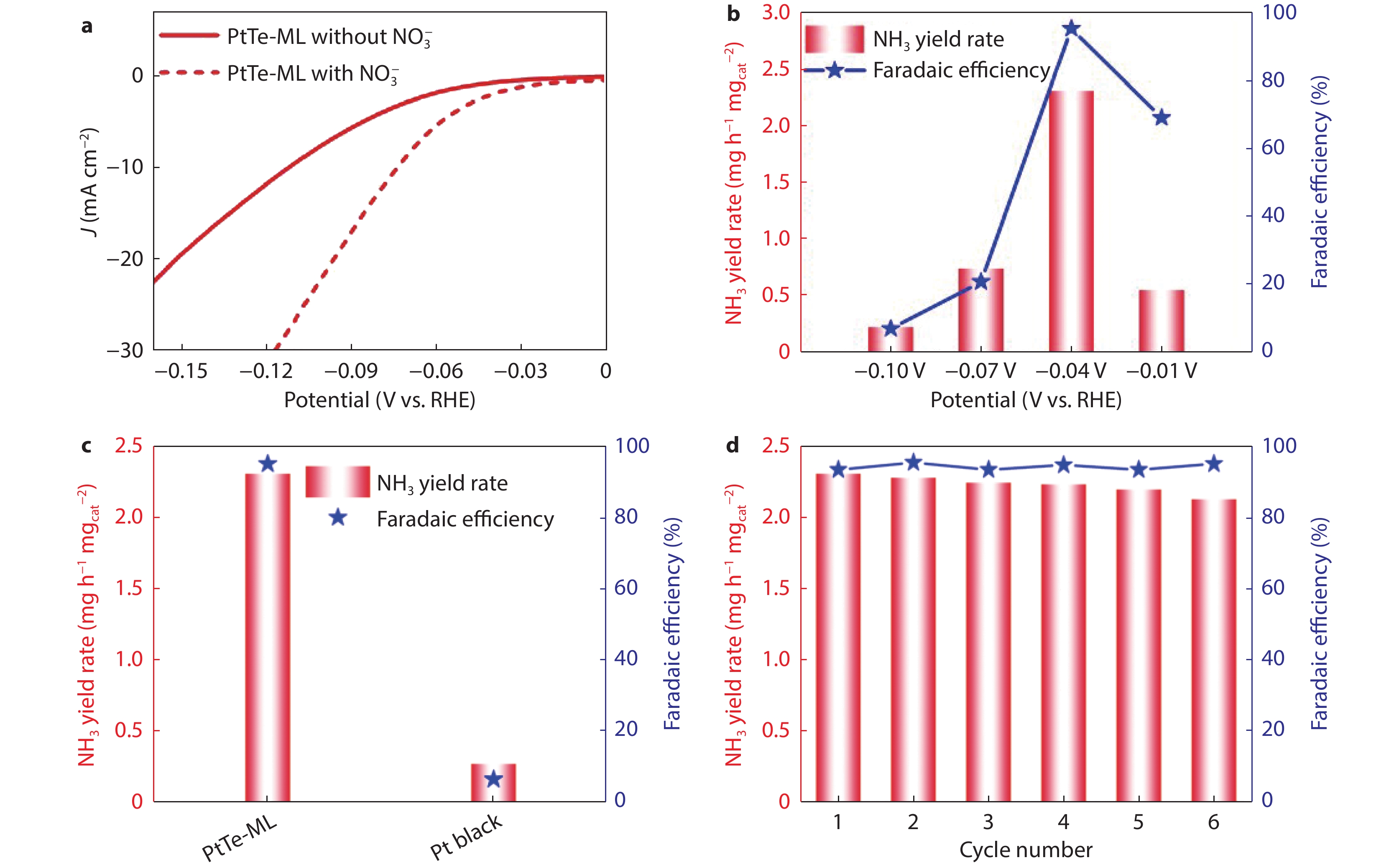
Figure 3.
a LSV curves of PtTe-ML in Ar-saturated 0.5 M H2SO4 electrolyte with and without 50 mM KNO3 at 50 mV s−1. b The Faradaic efficiency of NO3−-ERR and NH3 yield of PtTe-ML at different potentials. c NH3 yields and Faradaic efficiency of PtTe-ML and commercial Pt black at −0.04 V potential. d The as-obtained Faradaic efficiency and NH3 yield during cyclic stability test of PtTe-ML.
-
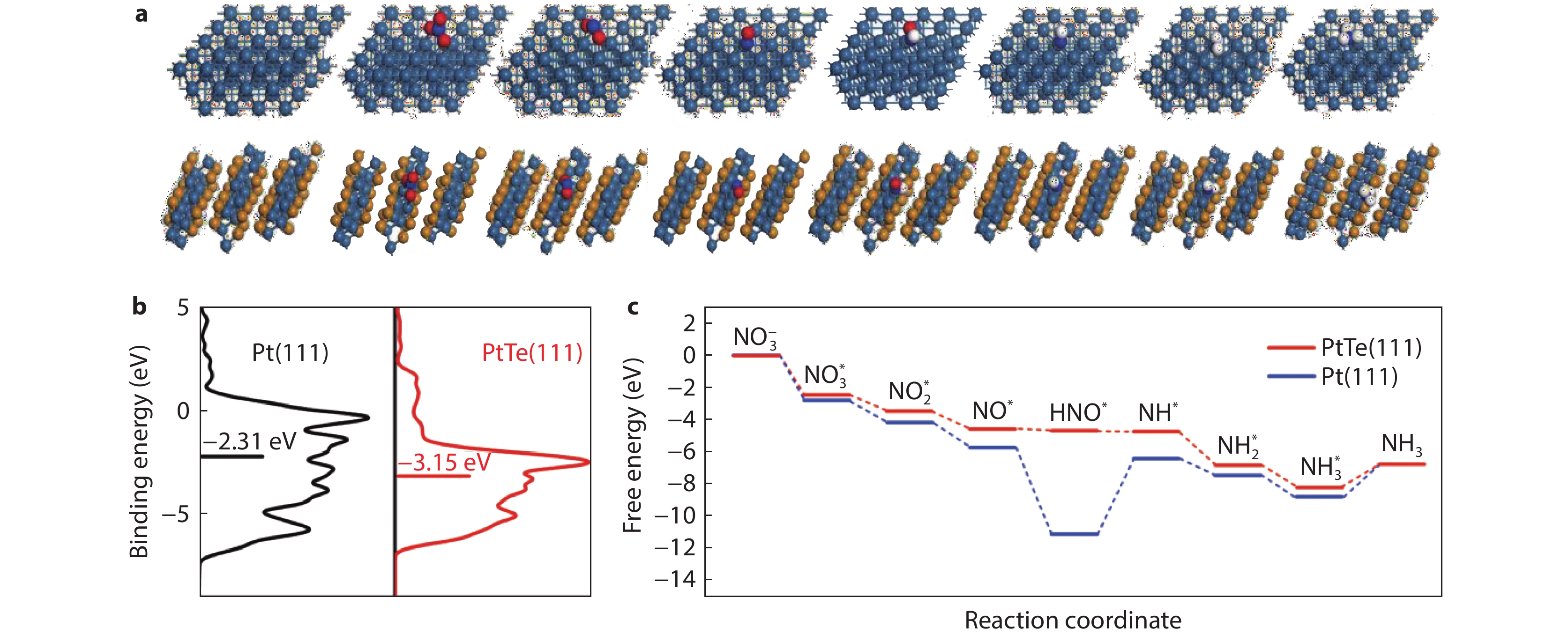
Figure 4.
a Atomic structure of the Pt(111) and PtTe(111) surface adsorbed with NO3* and intermediates. b Calculated d-band center values of PtTe(111) and Pt(111) surfaces. c The free energy diagrams of NO3−-ERR on the Pt(111) and PtTe(111) surfaces.
-
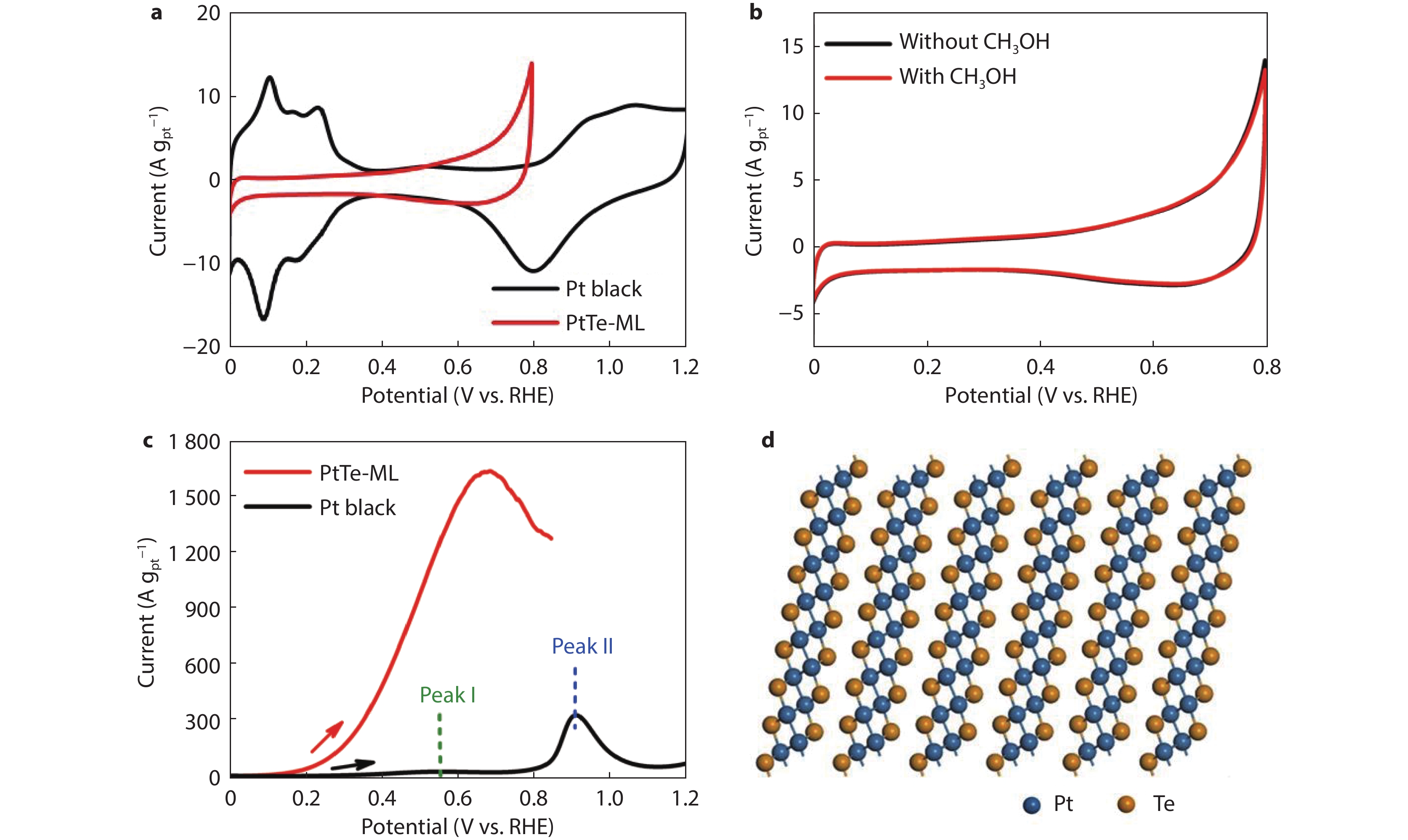
Figure 5.
a CV curves of PtTe-ML and commercial Pt balck in Ar-saturated 0.5 M H2SO4 electrolyte at 50 mV s−1. b CV curves of PtTe-ML in Ar-saturated 0.5 M H2SO4 electrolyte in the presence or absence of 0.5 M CH3OH at 50 mV s−1. c LSV curves of PtTe-ML and Pt balck in 0.5 M H2SO4 and 0.5 M HCOOH electrolyte at 50 mV s−1. d Atomic structure of PtTe-ML.
-
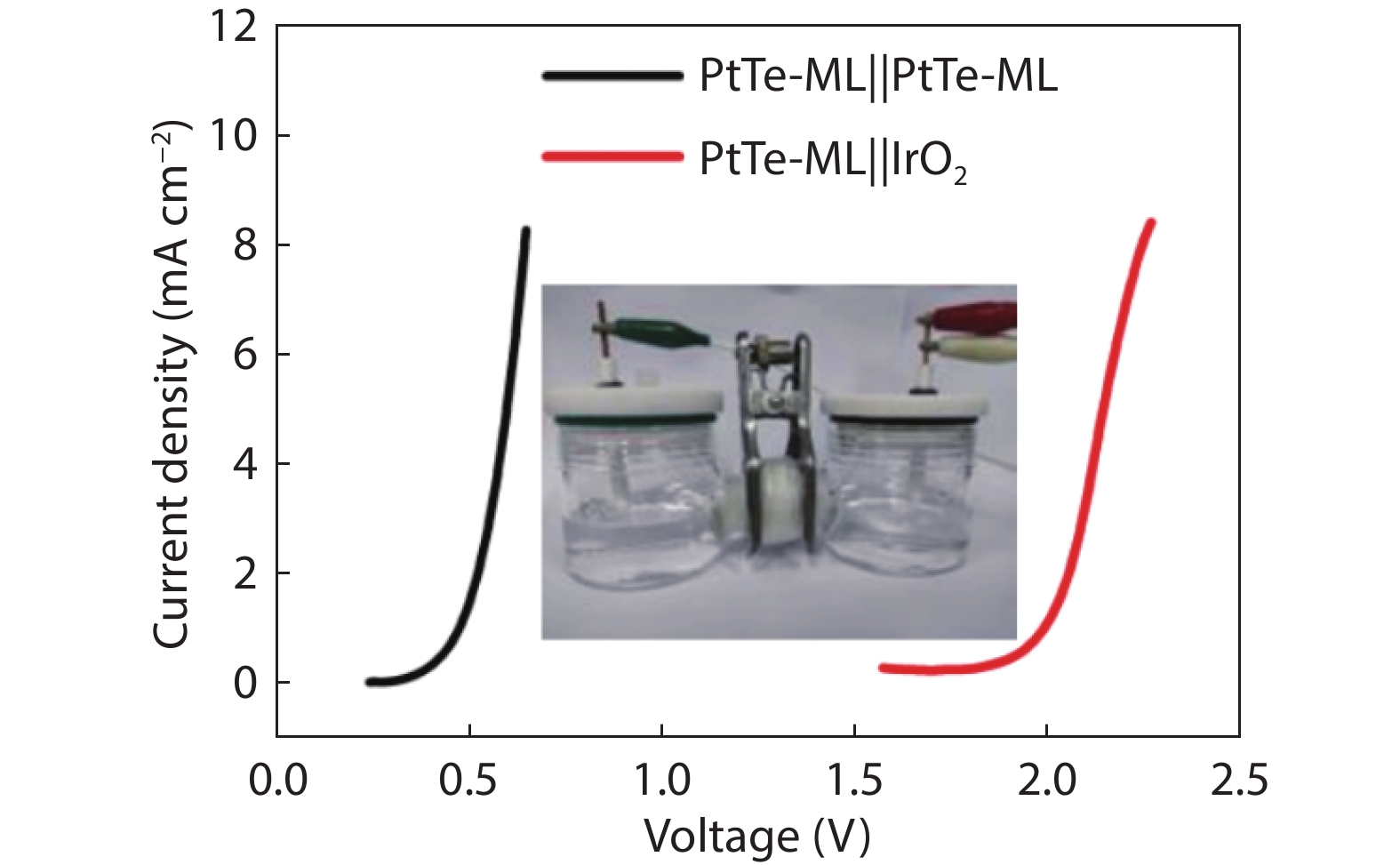
Figure 6.
Polarization curves of PtTe-ML||PtTe-ML electrolyzer in 0.5 M H2SO4 + 50 mM KNO3 + 0.5 M HCOOH solution and PtTe-ML||IrO2 electrolyzer in 0.5 M H2SO4.


 DownLoad:
DownLoad: