| Citation: | Yasen Hao, Xu Xiao, Zhuojun Zhang, Aijing Yan, Zehui Zhao, Tenghui Qiu, Peng Tan. Regulating reaction pathways in Hybrid-electrolyte Li-CO2 batteries via electrocatalytic CO2 reduction reaction paradigm migration[J]. Energy Lab. doi: 10.54227/elab.20250102 |
Regulating reaction pathways in Hybrid-electrolyte Li-CO2 batteries via electrocatalytic CO2 reduction reaction paradigm migration
-
Abstract
Owing to the high theoretical energy density, rechargeable lithium-carbon dioxide (Li-CO2) batteries attract significant attention for synergistic energy storage and carbon fixation. However, the performance of conventional single organic electrolyte systems is hindered by issues such as the deposition of insulating Li2CO3 and inefficient CO2 mass transport, which makes breakthroughs difficult. Hybrid-electrolyte systems isolate the anode and cathode environments via solid electrolytes, constructing an aqueous cathode to accommodate CO2 reduction reaction (CO2RR). This design enhances CO2 solubility, facilitates proton-coupled electron transfer, and suppresses solid-phase deposition, thereby significantly optimizing battery performance. Nevertheless, the cathode reaction mechanism remains unclear, with the evolution of key intermediates, proton/electron transfer pathways, and catalyst-electrolyte synergies yet to be clarified. This work elucidates the limitations of conventional systems, highlights the advantages of hybrid-electrolyte designs, and integrates established principles from CO2RR electrocatalysis, such as the regulation of reaction pathways by pH, salt concentration, and current density. This study aims to provide a theoretical framework for developing next-generation Li-CO2 batteries with high energy density and long cycle life while emphasizing the critical value of electrocatalytic insights in deepening the mechanistic understanding.
-
-
References
1. F. Wang, Y. Li, X. Xia, W. Cai, Q. Chen, M. Chen, Adv. Energy Mater., 2021, 11, 2100667. 2. X. Mu, H. Pan, P. He, H. Zhou, Adv. Mater., 2020, 32, 1903790. 3. S. J. Visco, E. Nimon, B. Katz, The Development of High Energy Density Lithium/Air and Lithium/Water Batteries with No Self-Discharge, ECS Meet. Abstr., 2006, MA2006-02, 389. 4. Y. Wang, H. Zhou, J. Power Sources, 2010, 195, 358. 5. R. Yang, Z. Peng, J. Xie, Y. Huang, R. A. Borse, X. Wang, M. Wu, Y. Wang, ChemSusChem, 2020, 13, 2621. 6. I. Bagemihl, C. Bhatraju, J. R. Van Ommen, V. Van Steijn, ACS Sustain. Chem. Eng., 2022, 10, 12580. 7. X. Xiao, Z. Zhang, P. Tan, Proc. Natl. Acad. Sci., 2023, 120, e2217454120. 8. Z. Zhang, X. Xiao, A. Yan, K. Sun, J. Yu, P. Tan, Nat. Commun., 2024, 15, 9952. 9. X. Xiao, Z. Zhang, A. Yan, L. Shi, P. Tan, Adv. Funct. Mater., 2025, 1, 2505676 10. L. Xiong, N. Q. Su, Inorg. Chem., 2025, 64, 8376. 11. X. Zhang, Y. Wang, Y. Li, J. Phys. Chem. Lett., 2023, 14, 1604. 12. X. Zhang, Y. Wang, Y. Li, Chem. Sci., 2024, 15, 4804. 13. J. M. Garcia-Lastra, J. S. G. Myrdal, R. Christensen, K. S. Thygesen, T. Vegge, DFT+U Study of Polaronic Conduction in Li2O2 and Li2CO3 : Implications for Li-Air Batteries, J. Phys. Chem. C, 2013, 117, 5568. 14. T. Yang, H. Li, J. Chen, H. Ye, J. Yao, Y. Su, B. Guo, Z. Peng, T. Shen, Y. Tang, L. Zhang, J. Huang, Nanoscale, 2020, 12, 23967. 15. P. Jia, M. Yu, X. Zhang, T. Yang, D. Zhu, T. Shen, L. Zhang, Y. Tang, J. Huang, Nano Res., 2022, 15, 542. 16. H. Wang, K. Xie, Y. You, Q. Hou, K. Zhang, N. Li, W. Yu, K. P. Loh, C. Shen, B. Wei, Adv. Energy Mater., 2019, 9, 1901806. 17. Z. Zhang, W. Bai, Z. Cai, J. Cheng, H. Kuang, B. Dong, Y. Wang, K. Wang, J. Chen, Angew. Chem. Int. Ed., 2021, 133, 16540. 18. Y. Bai, L. Wei, Y. Lian, Z. Wei, D. Song, Y. Su, X. Zhu, W. Huo, J. Cheng, Y. Peng, Z. Deng, ACS Appl. Mater. Interfaces, 2023, 15, 41457. 19. K. He, C. Zu, Y. Wang, B. Han, X. Yin, H. Zhao, Y. Liu, J. Chen, Solid State Ion., 2014, 254, 78. 20. H. Xue, H. Gong, X. Lu, B. Gao, T. Wang, J. He, Y. Yamauchi, T. Sasaki, R. Ma, Adv. Energy Mater., 2021, 11, 2101630. 21. X. Yang, D. Zhang, L. Zhao, C. Peng, K. Ren, C. Xu, P. Liu, Y. Zhou, Y. Lei, B. Yang, D. Xue, F. Liang, Adv. Energy Mater., 2024, 14, 2304365. 22. N. Feng, B. Wang, Z. Yu, Y. Gu, L. Xu, J. Ma, Y. Wang, Y. Xia, ACS Appl. Mater. Interfaces, 2021, 13, 7396. 23. Y. Li, Q. Yin, B. Jia, H. Wang, H. Gu, Q. Hu, H. Yang, T. Guo, P. Hu, L. Li, L. Liu, L. Guo, Angew. Chem. Int. Ed., 2025, 64, e202505668. 24. H. Ge, W. Shi, J. Liu, Y. Zhang, X. Wang, J. Am. Chem. Soc., 2025, 147, 8367. 25. J. Wu, F. G. Risalvato, P. P. Sharma, P. J. Pellechia, F. -S. Ke, X. -D. Zhou, J. Electrochem. Soc., 2013, 160, F953 26. Q. Wang, H. Dong, H. Yu, J. Power Sources, 2014, 271, 278. 27. J. Wu, P. P. Sharma, B. H. Harris, X. -D. Zhou, J. Power Sources, 2014, 258, 189 28. Q. Wang, H. Dong, H. Yu, H. Yu, J. Power Sources, 2015, 279, 1. 29. S. Ma, Y. Lan, G. M. J. Perez, S. Moniri, P. J. A. Kenis, ChemSusChem, 2014, 7, 866. 30. S. Verma, Y. Hamasaki, C. Kim, W. Huang, S. Lu, H. -R. M. Jhong, A. A. Gewirth, T. Fujigaya, N. Nakashima, P. J. A. Kenis, ACS Energy Lett., 2018, 3, 193 31. P. Bumroongsakulsawat, G. H. Kelsall, Electrochim. Acta, 2014, 141, 216. 32. T. T. H. Hoang, S. Verma, S. Ma, T. T. Fister, J. Timoshenko, A. I. Frenkel, P. J. A. Kenis, A. A. Gewirth, J. Am. Chem. Soc., 2018, 140, 5791. 33. B. Kim, S. Ma, H. -R. Molly Jhong, P. J. A. Kenis, Electrochim. Acta, 2015, 166, 271 34. X. Lu, D. Y. C. Leung, H. Wang, J. Xuan, Appl. Energy, 2017, 194, 549. 35. E. J. Dufek, T. E. Lister, M. E. McIlwain, J. Appl. Electrochem., 2011, 41, 623 36. Z. Jiang, Z. Zhang, H. Li, Y. Tang, Y. Yuan, J. Zao, H. Zheng, Y. Liang, Adv. Energy Mater., 2023, 13, 2203603. 37. X. Zhang, J. Li, Y. -Y. Li, Y. Jung, Y. Kuang, G. Zhu, Y. Liang, H. Dai, J. Am. Chem. Soc., 2021, 143, 3245 38. T. Yuan, T. Wang, G. Zhang, W. Deng, D. Cheng, H. Gao, J. Zhao, J. Yu, P. Zhang, J. Gong, Chem. Sci., 2022, 13, 8117. 39. H. “Molly” Jhong, F. R. Brushett, P. J. A. Kenis, Adv. Energy Mater., 2013, 3, 589. 40. Y. Kang, Y. Kim, Y. Doh, J. Lee, J. Kim, K. T. Park, Angew. Chem. Int. Ed., 2025, 137, e202504380. 41. C. G. Vayenas, R. E. White, M. E. Gamboa-Aldeco, Modern Aspects of Electrochemistry, Springer New York, New York, 2008. 42. G. Seshadri, C. Lin, A. B. Bocarsly, J. Electroanal. Chem., 1994, 372, 145. 43. A. S. Varela, W. Ju, T. Reier, P. Strasser, ACS Catal., 2016, 6, 2136. 44. F. P. García De Arquer, C. -T. Dinh, A. Ozden, J. Wicks, C. McCallum, A. R. Kirmani, D. -H. Nam, C. Gabardo, A. Seifitokaldani, X. Wang, Y. C. Li, F. Li, J. Edwards, L. J. Richter, S. J. Thorpe, D. Sinton, E. H. Sargent, Science, 2020, 367, 661 45. T. Burdyny, W. A. Smith, Energy Environ. Sci., 2019, 12, 1442. 46. M. König, J. Vaes, E. Klemm, D. Pant, iScience, 2019, 19, 135. 47. L. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang, K. Xu, Science, 2015, 350, 938. 48. Q. Dong, X. Zhang, D. He, C. Lang, D. Wang, ACS Cent. Sci., 2019, 5, 1461. 49. A. Martini, D. Hursán, J. Timoshenko, M. Rüscher, F. Haase, C. Rettenmaier, E. Ortega, A. Etxebarria, B. Roldan Cuenya, J. Am. Chem. Soc., 2023, 145, 17351. 50. H. Mistry, Y. Choi, A. Bagger, F. Scholten, C. S. Bonifacio, I. Sinev, N. J. Divins, I. Zegkinoglou, H. S. Jeon, K. Kisslinger, E. A. Stach, J. C. Yang, J. Rossmeisl, B. Roldan Cuenya, Angew. Chem. Int. Ed., 2017, 129, 11552. 51. Y. Liu, X. Fan, A. Nayak, Y. Wang, B. Shan, X. Quan, T. J. Meyer, Natl. Acad. Sci., 2019, 116, 26353. 52. F. Li, A. Thevenon, A. Rosas-Hernández, Z. Wang, Y. Li, C. M. Gabardo, A. Ozden, C. T. Dinh, J. Li, Y. Wang, J. P. Edwards, Y. Xu, C. McCallum, L. Tao, Z. -Q. Liang, M. Luo, X. Wang, H. Li, C. P. O’Brien, C. -S. Tan, D. -H. Nam, R. Quintero-Bermudez, T. -T. Zhuang, Y. C. Li, Z. Han, R. D. Britt, D. Sinton, T. Agapie, J. C. Peters, Nature, 2020, 577, 509 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Key evidence for the thermodynamics and reaction pathways of Li2CO3 formation. a Variation of decomposition free energy of Li2CO3 and Li2C2O4 with reaction steps. b, c Electronic band structures of Li2CO3 and Li2C2O4.[10] d Free energy curve for the formation of *Li2C2O4 in the first stage; the rate-determining step (C-C coupling) has an energy barrier of 0.81 eV. e Free energy and energy barriers (2.24-4.33 eV) for CO disproportionation 11 f CO2 adsorption structures on Ru (0001) and Ir (111) surfaces in DMSO solvent, with C-O bond lengths of 1.315-1.325 Å. g, h Energy barrier diagrams for CO2 disproportionation pathways (0.76-0.83 eV).[12]
-
Figure 2.
Path regulation of hybrid-electrolyte Li-CO2 batteries. a Schematic diagram of the porous Pd-based hybrid electrolyte battery structure. b Comparison of aqueous and conventional aprotic Li-CO2 batteries.[20] (c-d) XRD patterns of c Mo2C/CNT and d CNT cathodes. (e-f) XPS spectra of C 1s for the pristine, discharged, and recharged cathodes in WIS-based electrolyte: e Mo2C/CNT and f CNT cathode.[22]
-
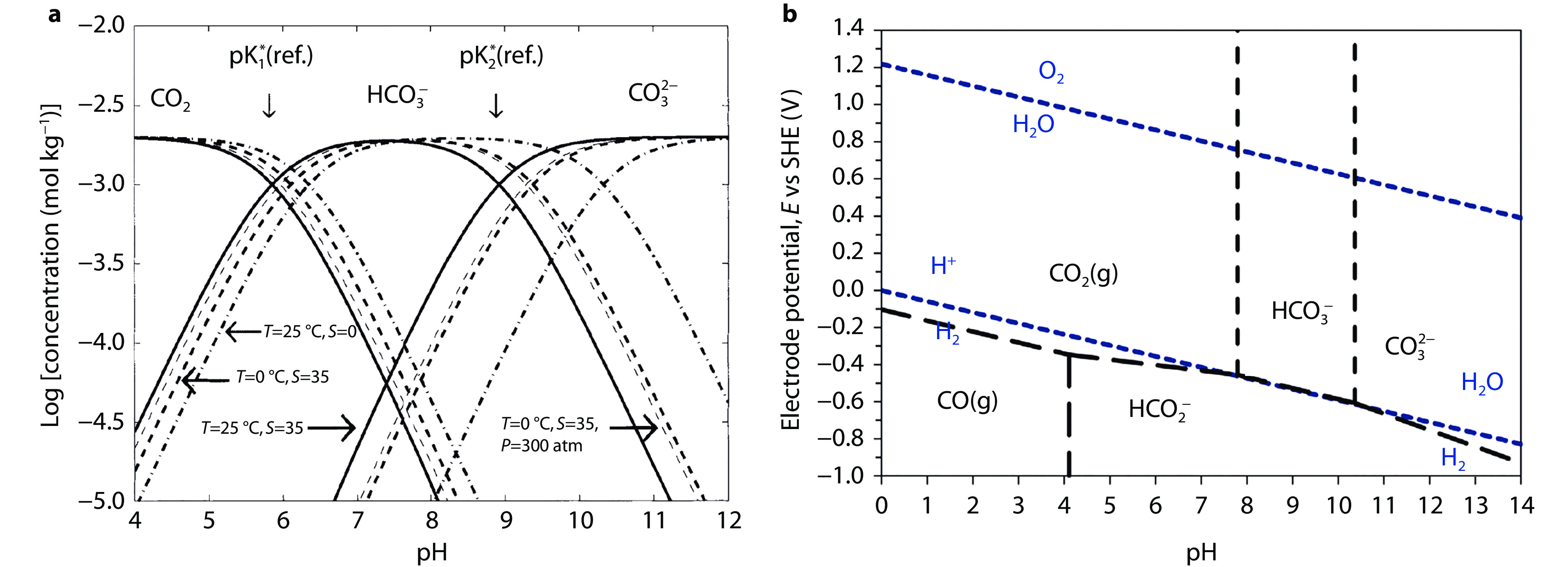
Figure 3.
Regulatory role of pH in key properties of CO2 in aqueous systems. a Solubility of CO2 in water as a function of the pH value at the indicated temperatures, salinities, and pressures. b Pourbaix diagram of CO2 and its related substances.[46]
-
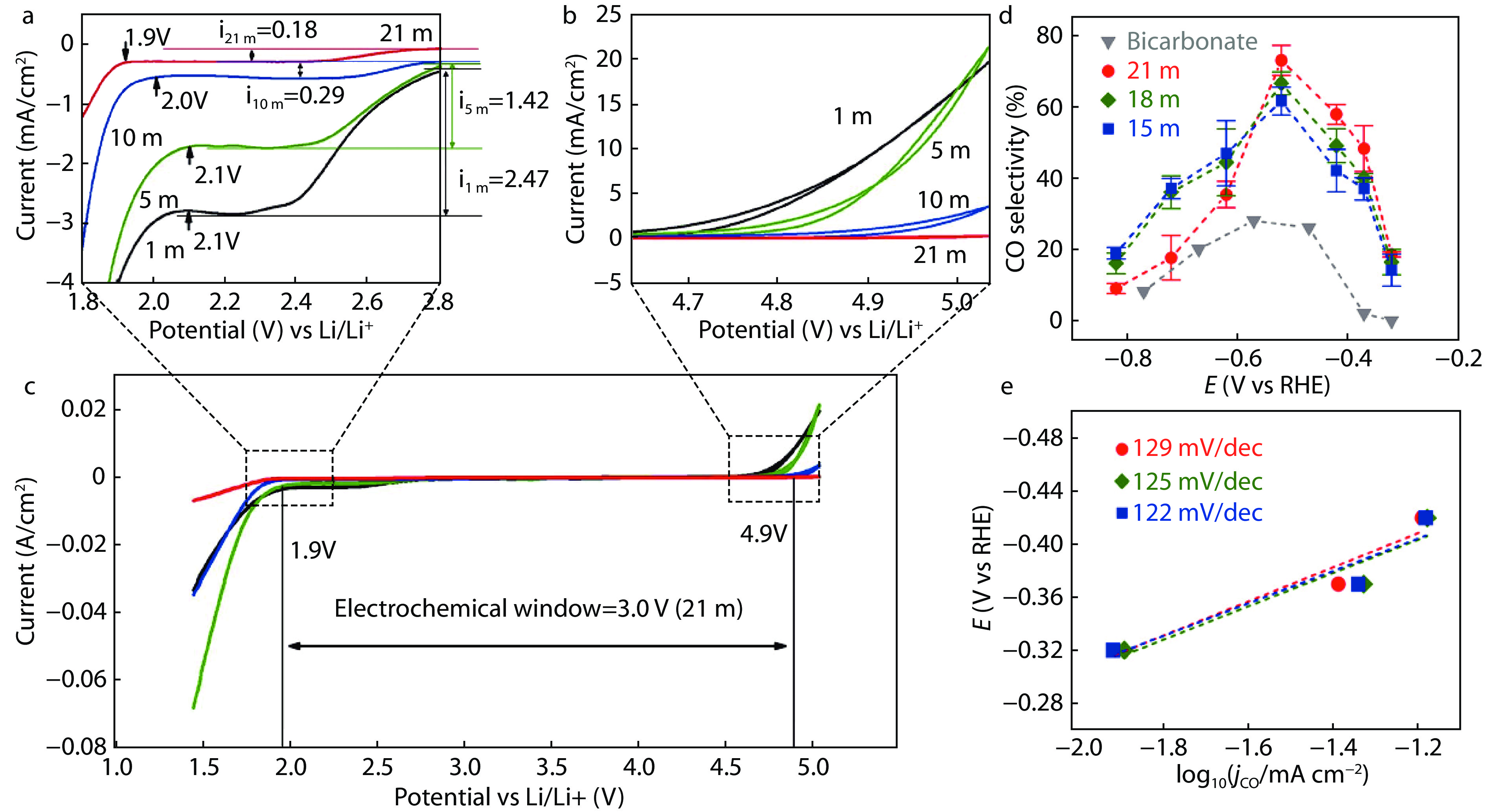
Figure 4.
Regulation of the electrochemical CO2RR by WIS electrolytes. a Overall electrochemical stability window. b and c Magnified view of the regions outlined near the anodic and cathodic extremes.[47] d All electrolytes were saturated with CO2. e Tafel analyses of WiS with different H2O concentrations.[48]
-
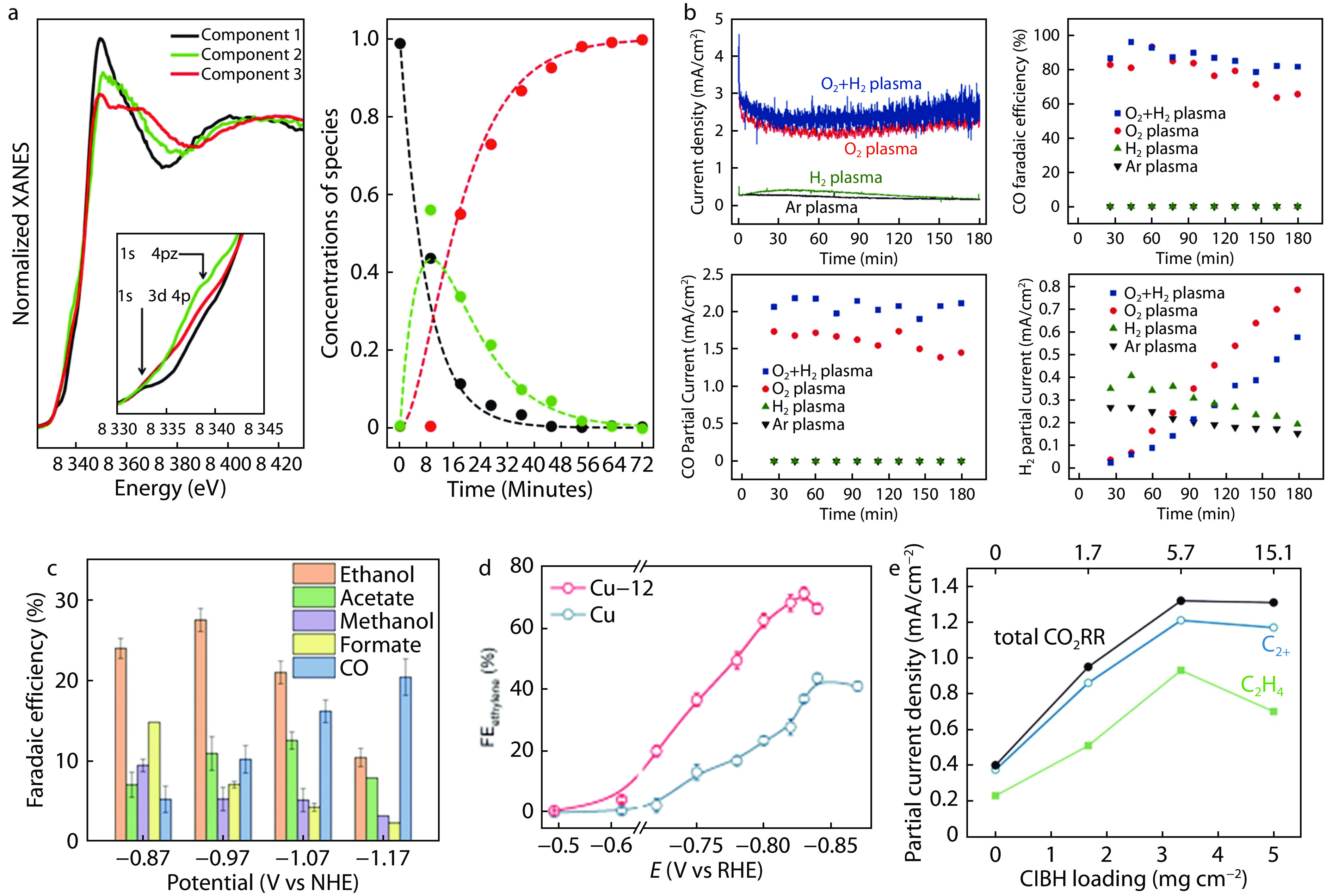
Figure 5.
Mechanisms Governing Current Density Regulation in the CO2RR. a XANES spectra for the extracted pure species and related concentration profiles (filled circles) extracted via the TM approach from the experimental Ni K-edge XANES data for the HTNi-TMNC sample.[49] b CO2 electroreduction over plasma-treated Ag foil catalysts at -0.6 Vvs. RHE in 0.1 m KHCO3.[50] c Faradaic efficiencies for ethanol, acetate, methanol, formate, and CO production on RuPC/NPC over a 1-h period.[51] d FE using CO2-saturated 1 M KHCO3 as the supporting electrolyte.[52] e Partial current density for total CO2RR reactions, with C2+ and C2H4 at the maximum cathodic energy efficiency.[44]
-
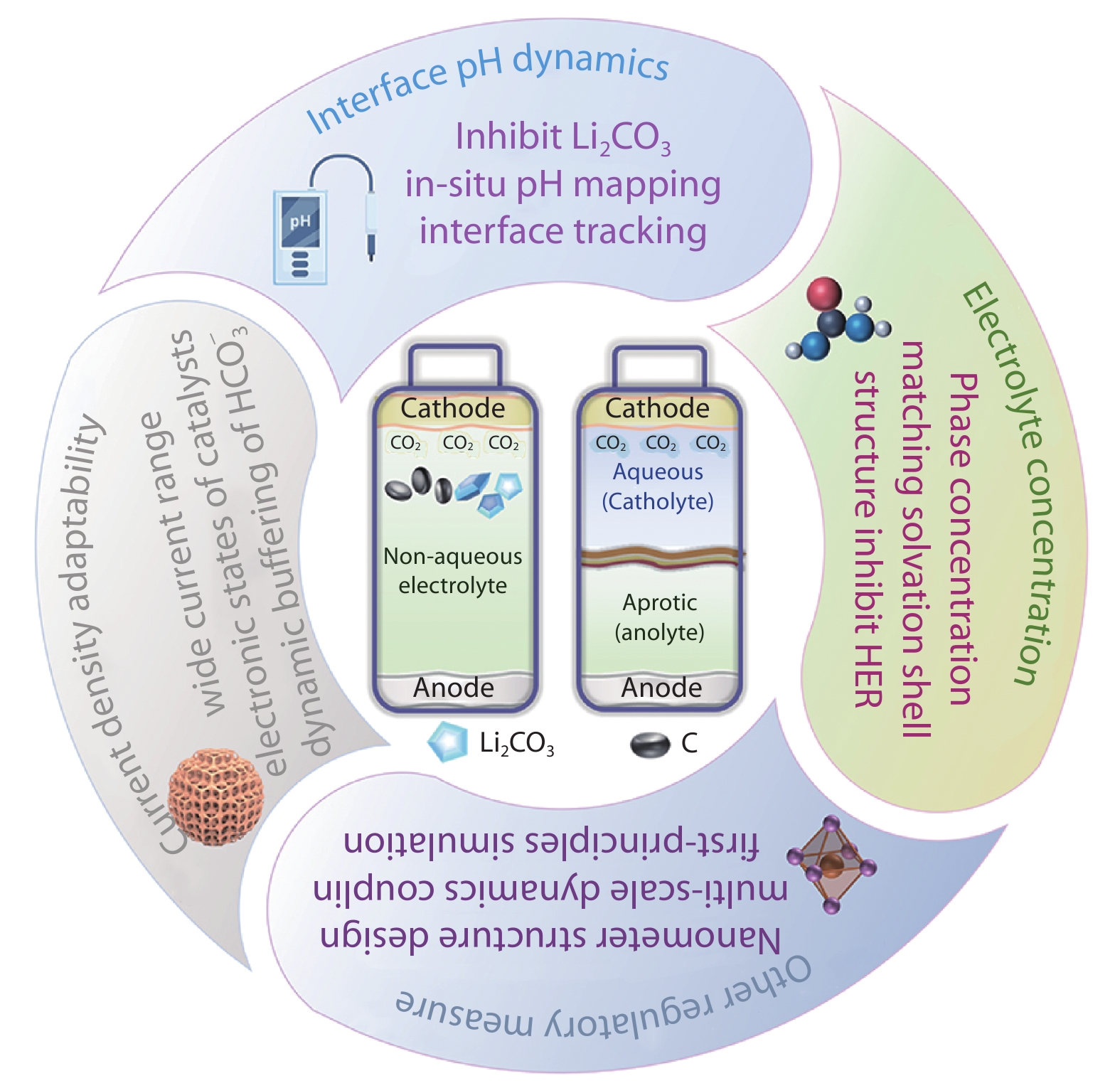
Figure 6.
Schematic illustration of multi-dimensional regulation strategies for hybrid electrolyte Li-CO2 batteries.

 Yasen Hao is a doctoral postgraduate student at the University of Science and Technology of China, with research interests concentrated on the transport mechanism and regulation strategies of hybrid-electrolyte lithium-carbon dioxide batteries.
Yasen Hao is a doctoral postgraduate student at the University of Science and Technology of China, with research interests concentrated on the transport mechanism and regulation strategies of hybrid-electrolyte lithium-carbon dioxide batteries.  Xu Xiao received her Ph.D. degree from the University of Science and Technology of China. She is currently an associate researcher at the University of Science and Technology of China. Her research interest mainly focuses on lithium-carbon dioxide batteries, including mechanism investigation, structure design, and modeling.
Xu Xiao received her Ph.D. degree from the University of Science and Technology of China. She is currently an associate researcher at the University of Science and Technology of China. Her research interest mainly focuses on lithium-carbon dioxide batteries, including mechanism investigation, structure design, and modeling.  Prof. Peng Tan received his PhD degree from Hong Kong University of Science and Technology. After a postdoctoral fellowship at Hong Kong Polytechnic University, he is currently a professor at University of Science and Technology of China. His research mainly focuses on the coupled species transfer and energy conversion inside batteries, with visualization technology for transport process observation, advanced models and simulations for analysis, and regulation strategies for performance improvement.
Prof. Peng Tan received his PhD degree from Hong Kong University of Science and Technology. After a postdoctoral fellowship at Hong Kong Polytechnic University, he is currently a professor at University of Science and Technology of China. His research mainly focuses on the coupled species transfer and energy conversion inside batteries, with visualization technology for transport process observation, advanced models and simulations for analysis, and regulation strategies for performance improvement. 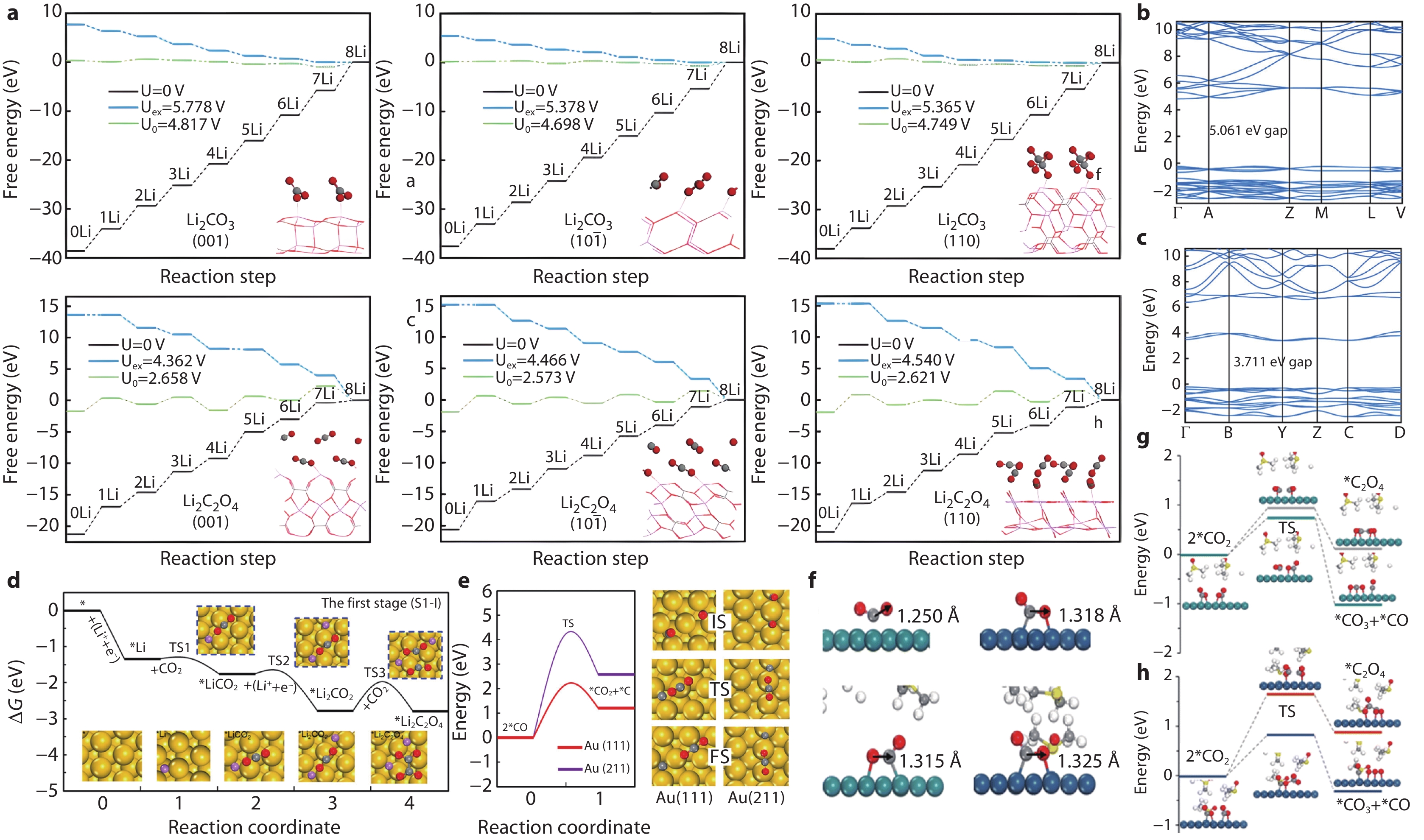

 DownLoad:
DownLoad: