| Citation: | Ruguang Ma, Xiaolin Zhao, Yu Pei, Yuyang Zong, Jianjun Liu, Jiacheng Wang. Modulating hydroxyl adsorption on transition metal nitrides by magnetic moments toward fast alkaline hydrogen evolution[J]. Energy Lab, 2024, 2(2): 230007. doi: 10.54227/elab.20230007 |
Modulating hydroxyl adsorption on transition metal nitrides by magnetic moments toward fast alkaline hydrogen evolution
-
Abstract
Water dissociation is a critical step limiting the kinetics of electrocatalytic hydrogen evolution reaction (HER) in the alkaline. However, the effect of hydroxyl groups (OH*) on the electrocatalyst surface during the HER process has not been clarified yet. Here, three typical transition metal (TM) nitrides (i.e., Fe2N, Co3N and Ni3N) were investigated by combing theoretical calculation and experiments toward alkaline HER. The results show binding energy of OH* (∆EOH*) and magnetic moment of three nitrides follow the same trend of Fe2N > Co3N > Ni3N, showing a positive correlation. The as-synthesized Ni3N with the smallest magnetic moment shows the best alkaline HER activity due to its lowest water-dissociation barrier and weakest ∆EOH*. Weak adsorption to OH* could promote the fast release of OH* and thus enhance reaction kinetics. A small bond order corresponding to weak interaction between Ni3N and OH* is found to be favorable to the release of OH* and subsequent reaction steps, which originates from the orbital interaction of outer-shell electrons in TM and OH*. This work provides new insights into the design of advanced electrocatalysts using magnetic matrix toward alkaline HER.
-
Keywords:
- Water dissociation /
- Magnetic moment /
- Bond order /
- Hydroxyl group /
- Hydrogen evolution reaction
-
-
References
1. Y. Zhou and H. J. Fan, ACS Mater. Lett., 2021, 3, 136 2. I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem., 2017, 1, 0003 3. R. Subbaraman, D. Tripkovic, D. Strmcnik, K.-C. Chang, M. Uchimura, A. P. Paulikas, V. Stamenkovic and N. M. Markovic, Science, 2011, 334, 1256 4. N. Danilovic, R. Subbaraman, D. Strmcnik, K.-C. Chang, A. P. Paulikas, V. R. Stamenkovic and N. M. Markovic, Angew. Chem. Int. Ed., 2012, 124, 12663 5. B. Zhang, J. Liu, J. Wang, Y. Ruan, X. Ji, K. Xu, C. Chen, H. Wan, L. Miao and J. Jiang, Nano Energy, 2017, 37, 74 6. J. Huang, J. Han, T. Wu, K. Feng, T. Yao, X. Wang, S. Liu, J. Zhong, Z. Zhang, Y. Zhang and B. Song, ACS Energy Lett., 2019, 4, 3002 7. Z. Li, Y. Pei, R. Ma, Y. Wang, Y. Zhu, M. Yang and J. Wang, J. Mater. Chem. A, 2021, 9, 13109 8. K. Lu, Y. Liu, F. Lin, I. A. Cordova, S. Gao, B. Li, B. Peng, H. Xu, J. Kaelin, D. Coliz, C. Wang, Y. Shao and Y. Cheng, J. Am. Chem. Soc., 2020, 142, 12613 9. J. Qian, X. Wang, H. Jiang, S. Li, C. Li, S. Li, R. Ma and J. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 18607 10. Y. Dou, D. Yuan, L. Yu, W. Zhang, L. Zhang, K. Fan, M. Al-Mamun, P. Liu, C. -T. He and H. Zhao, Adv. Mater., 2022, 34, 2104667 11. S. Li, Z. Li, R. Ma, C. Gao, L. Liu, L. Hu, J. Zhu, T. Sun, Y. Tang, D. Liu and J. Wang, Angew. Chem. Int. Ed., 2021, 60, 3773 12. J. Xie and Y. Xie, Chem. Eur. J., 2016, 22, 3588 13. N. Han, P. Liu, J. Jiang, L. Ai, Z. Shao and S. Liu, J. Mater. Chem. A, 2018, 6, 19912 14. J. K. Nørskov, F. Abild-Pedersen, F. Studt and T. Bligaard, Proc. Natl. Acad. Sci., 2011, 108, 937 15. X. Jia, Y. Zhao, G. Chen, L. Shang, R. Shi, X. Kang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Energy Mater., 2016, 6, 1502585 16. G. Zhou, P. Wang, H. Li, B. Hu, Y. Sun, R. Huang and L. Liu, Nat. Commun., 2021, 12, 4827 17. X. Li, H. Liu, Z. Chen, Q. Wu, Z. Yu, M. Yang, X. Wang, Z. Cheng, Z. Fu and Y. Lu, Nat. Commun., 2019, 10, 1409 18. G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15 19. G. Kresse and J. Furthmüller, Physical Review B, 1996, 54, 11169 20. J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 21. G. Kresse and D. Joubert, Phys. Rev. B, 1999, 59, 1758 22. I. A. Vladimir, F. Aryasetiawan and A. I. Lichtenstein, J. Phys. Condens. Mat., 1997, 9, 767 23. S. Watanabe, Y. Sawada, M. Nakaya, M. Yoshino, T. Nagasaki, T. Kameyama, T. Torimoto, Y. Inaba, H. Takahashi, K. Takeshita and J. Onoe, J. Appl. Phys., 2016, 119, 235102 24. X. Zhao, Z. Hu, Y. Li, Y. Wang, E. Song, L. Zhang and J. Liu, Mater. Horiz., 2021, 8, 1825 25. P. Błoński and J. Hafner, J. Phys. Condens. Mat., 2009, 21, 426001 26. G. Henkelman, B. P. Uberuaga and H. Jónsson, J. Chem. Phys., 2000, 113, 9901 27. J. K. Nørskov, T. Bligaard, A. Logadottir, J. R. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 28. Y. Pei, B. Rezaei, X. Zhang, Z. Li, H. Shen, M. Yang and J. Wang, Mater. Chem. Front., 2020, 4, 2665 29. J. Greeley, T. F. Jaramillo, J. Bonde, I. Chorkendorff and J. K. Nørskov, Nat. Mater., 2006, 5, 909 30. A. P. Grosvenor, B. A. Kobe, M. C. Biesinger and N. S. McIntyre, Surf. Interface Anal., 2004, 36, 1564 31. F. Yan, Y. Wang, K. Li, C. Zhu, P. Gao, C. Li, X. Zhang and Y. Chen, Chem. Eur. J., 2017, 23, 10187 32. X. Li, Z. Ao, J. Liu, H. Sun, A. I. Rykov and J. Wang, ACS Nano, 2016, 10, 11532 33. Y. Hu, H. Duan, J., Y. Huang, M. Balogun, Y. Tong, ChemCatChem, 2019, 11, 6051 34. D. Zhang, H. Li, Asim R., Astha S., W. Liang, Y. Wang, H. Chen, Kaushal V., D. Yan, Zhen S., Antonio T., C. Zhao, F. Beck, Karsten R., K. Catchpole, Siva K, Energy Environ. Sci., 2022, 15, 185 35. M. Lao, P. Li, Y. Jiang, H. Pan, S. X. Dou and W. Sun, Nano Energy, 2022, 98, 107231 36. Y. Sun, S. Sun, H. Yang, S. Xi, J. Gracia and Z. J. Xu, Adv. Mater., 2020, 32, 2003297 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Structural models of a Fe2N, b Co3N and c Ni3N; pDOS of d Fe2N, e Co3N and f Ni3N before and after adsorption of hydroxyl group (OH*); g Gibbs free energy change (∆GH*) of H* and binding energy of OH* (∆EOH*) on the surface of Co3N, Ni3N and Fe2N. h Bader charge of Fe2N, Co3N and Ni3N. i The relationship between the calculated magnetic moment and ∆EOH* for Fe2N, Co3N and Ni3N.
-
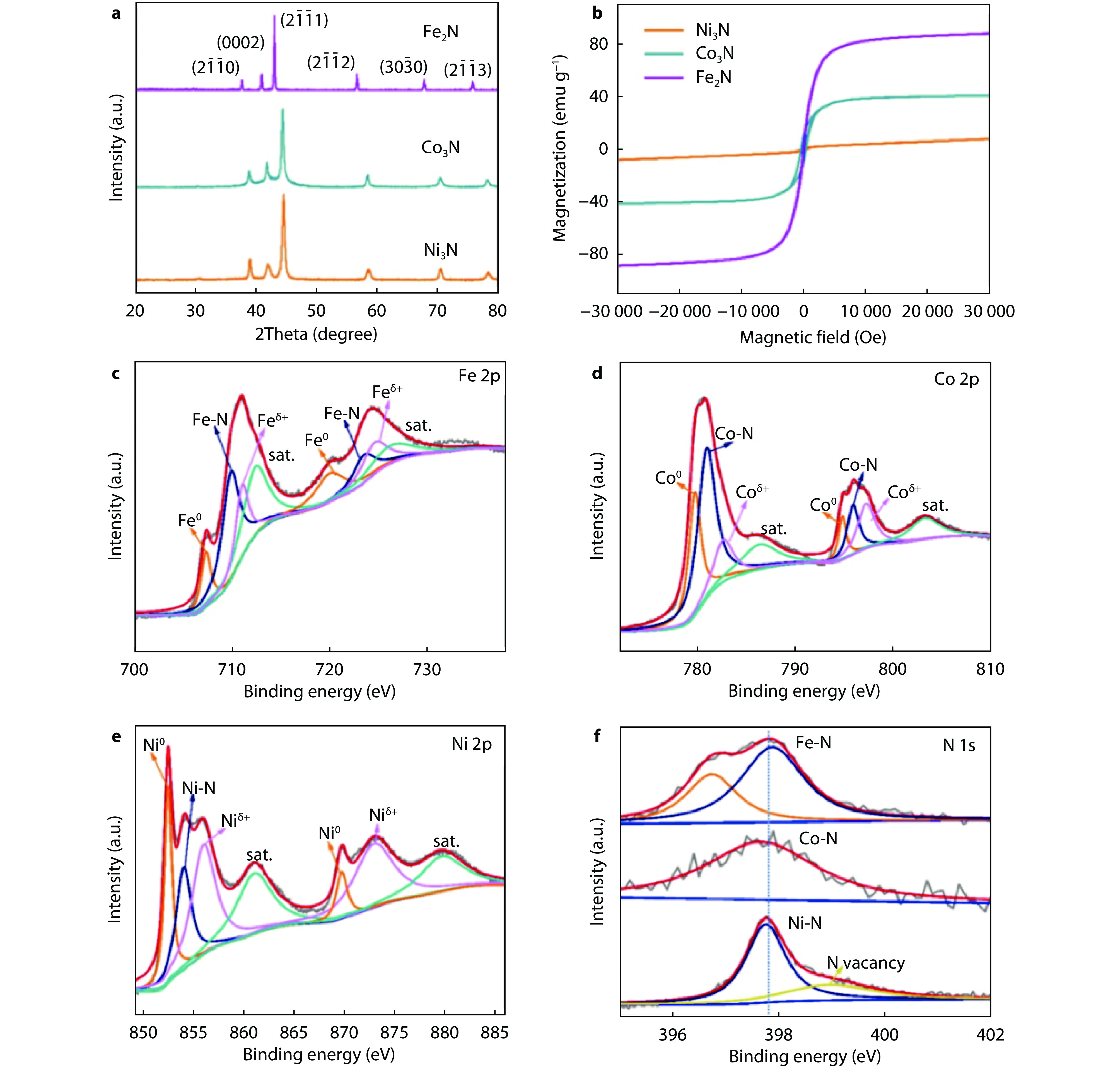
Figure 2.
a XRD patterns, b magnetization hysteresis loops M(H) of Fe2N, Co3N and Ni3N at room temperature. c-f High-resolution XPS of c Fe 2p, d Co 2p, e Ni 2p and f N 1s in Fe2N, Co3N and Ni3N, respectively.
-
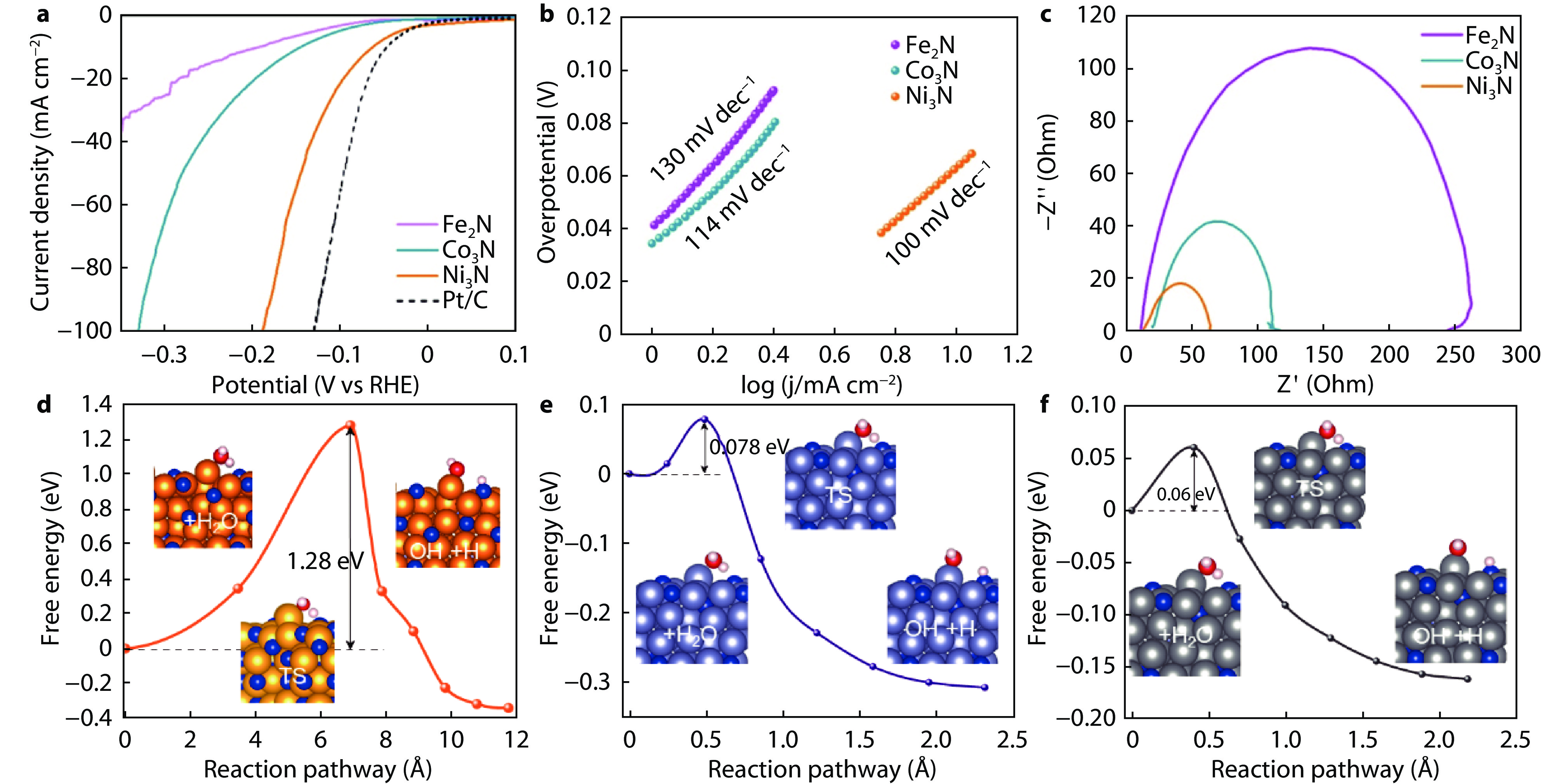
Figure 3.
a LSV curves, b Tafel slope, c EIS of Fe2N, Co3N and Ni3N, respectively. The energy profile of water dissociation on the surface of d Fe2N, e Co3N and f Ni3N. The insets show the corresponding surfaces with H2O adsorption, H and OH co-adsorption.
-
Figure 4.
Schematic illustration of orbital interaction of outer-shell electrons between OH− and a Fe2N, b Co3N and c Ni3N. The corresponding water splitting process on different spin state surface is also displayed.


 DownLoad:
DownLoad: