| Citation: | Yujia Long, Ruitao Lv. Degradation mechanisms and rational design of durable oxygen evolution electrocatalysts under acidic conditions[J]. Energy Lab, 2025, 3(2): 250005. doi: 10.54227/elab.20250005 |
Degradation mechanisms and rational design of durable oxygen evolution electrocatalysts under acidic conditions
-
Abstract
Proton exchange membrane water electrolysis (PEMWE) stands as a pivotal technology for scalable green hydrogen production, yet its efficiency is hindered by the instability of oxygen evolution reaction (OER) catalysts under acidic and oxidative conditions. This review article systematically examines the degradation mechanisms of OER electrocatalysts in PEMWE, categorizing them into intrinsic and extrinsic factors. Intrinsic degradation arises from active metal dissolution (e.g., Ru/Ir oxidative leaching), dynamic surface reconstruction, and structural collapse induced by lattice oxygen participation via the lattice oxygen oxidation mechanism (LOM). Extrinsic instability stems from corrosion-prone supports (e.g., carbon-based materials or oxidized Ti substrates) and weakened catalyst-support interactions due to binder degradation or bubble-induced detachment. To address these challenges, advanced strategies are discussed, including electronic modulation of active sites (e.g., alloying, doping), rational utilization of surface reconstruction, suppression of LOM pathways, and the integration of corrosion-resistant supports with optimized metal-support interactions. Furthermore, self-supported catalysts and superaerophobic electrode designs are highlighted to mitigate delamination. Finally, critical research gaps are identified, emphasizing the need for standardized stability evaluation protocols, in-situ/operando characterization techniques, and holistic integration of catalyst design with PEMWE system components (e.g., membranes, flow fields) to bridge laboratory advancements with industrial-scale applications.
-
-
References
1. Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov, T. F. Jaramillo, Science, 2017, 355, eaad4998 2. C. Yu, M. Moslehpour, T. K. Tran, L. M. Trung, J. P. Ou, N. H. Tien, Resour. Policy, 2023, 80, 103221 3. P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo, E. H. Sargent, Science, 2019, 364, eaav3506 4. M. Rastgar, K. Moradi, C. Burroughs, A. Hemmati, E. Hoek, M. Sadrzadeh, Chem. Rev., 2023, 123, 10156 5. Z. Huang, R. Lu, Y. Zhang, W. Chen, G. Chen, C. Ma, Z. Wang, Y. Han, W. Huang, Adv. Funct. Mater., 2023, 33, 2306333 6. J. A. Turner, Science, 2004, 305, 972 7. Z. Cui, W. Jiao, Z. Huang, G. Chen, B. Zhang, Y. Han, W. Huang, Small, 2023, 19, 2301465 8. L. Li, G. Zhang, C. Zhou, F. Lv, Y. Tan, Y. Han, H. Luo, D. Wang, Y. Liu, C. Shang, L. Zeng, Q. Huang, R. Zeng, N. Ye, M. Luo, S. Guo, Nat. Commun., 2024, 15, 4974 9. T. Wang, X. Cao, L. Jiao, Angew. Chem. Int. Ed., 2022, 61, e202213328 10. D. Chen, R. Yu, K. Yu, R. Lu, H. Zhao, J. Jiao, Y. Yao, J. Zhu, J. Wu, S. Mu, Nat. Commun., 2024, 15, 3928 11. Z. Shi, J. Li, J. Jiang, Y. Wang, X. Wang, Y. Li, L. Yang, Y. Chu, J. Bai, J. Yang, J. Ni, Y. Wang, L. Zhang, Z. Jiang, C. Liu, J. Ge, W. Xing, Angew. Chem. Int. Ed., 2022, 61, e202212341 12. H. Liu, Z. Zhang, J. Fang, M. Li, M. G. Sendeku, X. Wang, H. Wu, Y. Li, J. Ge, Z. Zhuang, D. Zhou, Y. Kuang, X. Sun, Joule, 2023, 7, 558 13. M. Carmo, D. L. Fritz, J. Mergel, D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901 14. L. Chong, G. Gao, J. Wen, H. Li, H. Xu, Z. Green, J. D. Sugar, A. J. Kropf, W. Xu, X.-M. Lin, H. Xu, L.-W. Wang, D.-J. Liu, Science, 2023, 380, 609 15. B. Lu, C. Wahl, R. dos Reis, J. Edgington, X. K. Lu, R. Li, M. E. Sweers, B. Ruggiero, G. T. K. K. Gunasooriya, V. Dravid, L. C. Seitz, Nat. Catal., 2024, 7, 868 16. I. C. Man, H.-Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov, J. Rossmeisl, ChemCatChem, 2011, 3, 1159 17. S. Wang, T. Shen, C. Yang, G. Luo, D. Wang, ACS Catal., 2023, 13, 8670 18. J. Torrero, T. Morawietz, D. García Sanchez, D. Galyamin, M. Retuerto, V. Martin-Diaconescu, S. Rojas, J. A. Alonso, A. S. Gago, K. A. Friedrich, Adv. Energy Mater., 2023, 13, 2204169 19. Z. Wu, Y. Wang, D. Liu, B. Zhou, P. Yang, R. Liu, W. Xiao, T. Ma, J. Wang, L. Wang, Adv. Funct. Mater., 2023, 33, 2307010 20. H. Xie, Z. Zhao, T. Liu, Y. Wu, C. Lan, W. Jiang, L. Zhu, Y. Wang, D. Yang, Z. Shao, Nature, 2022, 612, 673 21. L. Deng, S. Liu, D. Liu, Y.-M. Chang, L. Li, C. Li, Y. Sun, F. Hu, H.-Y. Chen, H. Pan, S. Peng, Small, 2023, 19, 2302238 22. K. A. Stoerzinger, O. Diaz-Morales, M. Kolb, R. R. Rao, R. Frydendal, L. Qiao, X. R. Wang, N. B. Halck, J. Rossmeisl, H. A. Hansen, T. Vegge, I. E. L. Stephens, M. T. M. Koper, Y. Shao-Horn, ACS Energy Lett., 2017, 2, 876 23. H. Wang, P. Yang, X. Sun, W. Xiao, X. Wang, M. Tian, G. Xu, Z. Li, Y. Zhang, F. Liu, L. Wang, Z. Wu, J. Energy Chem., 2023, 87, 286 24. D. Zhang, M. Li, X. Yong, H. Song, G. I. N. Waterhouse, Y. Yi, B. Xue, D. Zhang, B. Liu, S. Lu, Nat. Commun., 2023, 14, 2517 25. Y. Wang, K. Wei, Y. Song, A. A. Obisanya, H. Li, J. Wang, H. Li, F. Gao, Chem. Eng. J., 2024, 497, 154724 26. W. He, X. Tan, Y. Guo, Y. Xiao, H. Cui, C. Wang, Angew. Chem. Int. Ed., 2024, 63, e202405798 27. R. Kötz, S. Stucki, D. Scherson, D. M. Kolb, J. Electroanal. Chem. Interfacial Electrochem., 1984, 172, 211 28. Y. Matsumoto and E. Sato, Mater. Chem. Phys., 1986, 14, 397 29. T. Reier, H. N. Nong, D. Teschner, R. Schlögl, P. Strasser, Adv. Energy Mater., 2017, 7, 1601275 30. Y. Shi, H. Wu, J. Chang, Z. Tang, S. Lu, J. Energy Chem., 2023, 85, 220 31. J. Rossmeisl, A. Logadottir, J. K. Nørskov, Chem. Phys., 2005, 319, 178 32. J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes, J. K. Nørskov, J. Electroanal. Chem., 2007, 607, 83 33. M. T. M. Koper, Chem. Sci., 2013, 4, 2710 34. J. Shan, T. Ling, K. Davey, Y. Zheng, S.-Z. Qiao, Adv. Mater., 2019, 31, 1900510 35. H. Dau, C. Limberg, T. Reier, M. Risch, S. Roggan, P. Strasser, ChemCatChem, 2010, 2, 724 36. J. Rossmeisl, K. Dimitrievski, P. Siegbahn, J. K. Nørskov, J. Phys. Chem. C, 2007, 111, 18821 37. M. T. M. Koper, J. Electroanal. Chem., 2011, 660, 254 38. P. Sabatier, Ber. Dtsch. Chem. Ges., 1911, 44, 1984 39. A. Grimaud, A. Demortière, M. Saubanère, W. Dachraoui, M. Duchamp, M.-L. Doublet, J.-M. Tarascon, Nat. Energy, 2017, 2, 16189 40. A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y.-L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper, Y. Shao-Horn, Nat. Chem., 2017, 9, 457 41. H. Su, C. Yang, M. Liu, X. Zhang, W. Zhou, Y. Zhang, K. Zheng, S. Lian, Q. Liu, Nat. Commun., 2024, 15, 95 42. E. Fabbri and T. J. Schmidt, ACS Catal., 2018, 8, 9765 43. Z. Shi, Y. Wang, J. Li, X. Wang, Y. Wang, Y. Li, W. Xu, Z. Jiang, C. Liu, W. Xing, J. Ge, Joule, 2021, 5, 2164 44. D. A. Kuznetsov, M. A. Naeem, P. V. Kumar, P. M. Abdala, A. Fedorov, C. R. Müller, J. Am. Chem. Soc., 2020, 142, 7883 45. J. S. Yoo, X. Rong, Y. Liu, A. M. Kolpak, ACS Catal., 2018, 8, 4628 46. S. Hao, M. Liu, J. Pan, X. Liu, X. Tan, N. Xu, Y. He, L. Lei, X. Zhang, Nat. Commun., 2020, 11, 5368 47. M. Okamura, M. Kondo, R. Kuga, Y. Kurashige, T. Yanai, S. Hayami, V. K. K. Praneeth, M. Yoshida, K. Yoneda, S. Kawata, S. Masaoka, Nature, 2016, 530, 465 48. C. Lin, J.-L. Li, X. Li, S. Yang, W. Luo, Y. Zhang, S.-H. Kim, D.-H. Kim, S. S. Shinde, Y.-F. Li, Z.-P. Liu, Z. Jiang, J.-H. Lee, Nat. Catal., 2021, 4, 1012 49. J. Chang, Y. Shi, H. Wu, J. Yu, W. Jing, S. Wang, G. I. N. Waterhouse, Z. Tang, S. Lu, J. Am. Chem. Soc., 2024, 146, 12958 50. A. Zagalskaya and V. Alexandrov, ACS Catal., 2020, 10, 3650 51. J. Edgington and L. C. Seitz, ACS Catal., 2023, 13, 3379 52. F. Zeng, C. Mebrahtu, L. Liao, A. K. Beine, R. Palkovits, J. Energy Chem., 2022, 69, 301 53. N. Danilovic, R. Subbaraman, K.-C. Chang, S. H. Chang, Y. J. Kang, J. Snyder, A. P. Paulikas, D. Strmcnik, Y.-T. Kim, D. Myers, V. R. Stamenkovic, N. M. Markovic, J. Phys. Chem. Lett., 2014, 5, 2474 54. D. Hochfilzer, I. Chorkendorff, J. Kibsgaard, ACS Energy Lett., 2023, 8, 1607 55. S. Cherevko, A. R. Zeradjanin, A. A. Topalov, N. Kulyk, I. Katsounaros, K. J. J. Mayrhofer, ChemCatChem, 2014, 6, 2219 56. Z. Wang, X. Guo, J. Montoya, J. K. Nørskov, npj Comput. Mater., 2020, 6, 160 57. Z. Wang, Y.-R. Zheng, I. Chorkendorff, J. K. Nørskov, ACS Energy Lett., 2020, 5, 2905 58. L. Tang, B. Han, K. Persson, C. Friesen, T. He, K. Sieradzki, G. Ceder, J Am Chem Soc., 2010, 132, 596 59. S. Geiger, O. Kasian, B. R. Shrestha, A. M. Mingers, K. J. J. Mayrhofer, S. Cherevko, J. Electrochem. Soc., 2016, 163, F3132 60. M. A. Hubert, A. M. Patel, A. Gallo, Y. Liu, E. Valle, M. Ben-Naim, J. Sanchez, D. Sokaras, R. Sinclair, J. K. Nørskov, L. A. King, M. Bajdich, T. F. Jaramillo, ACS Catal., 2020, 10, 12182 61. Z. Kou, X. Li, L. Zhang, W. Zang, X. Gao, J. Wang, Small Sci., 2021, 1, 2170017 62. Y. Zeng, M. Zhao, Z. Huang, W. Zhu, J. Zheng, Q. Jiang, Z. Wang, H. Liang, Adv. Energy Mater., 2022, 12, 2201713 63. H. Zhong, Q. Zhang, J. Yu, X. Zhang, C. Wu, Y. Ma, H. An, H. Wang, J. Zhang, X. Wang, J. Xue, Adv. Energy Mater., 2023, 13, 2301391 64. M. Kim, J. Park, M. Wang, Q. Wang, M. J. Kim, J. Y. Kim, H.-S. Cho, C.-H. Kim, Z. Feng, B.-H. Kim, S. W. Lee, Appl. Catal. B, 2022, 302, 120834 65. J. Edgington, N. Schweitzer, S. Alayoglu, L. C. Seitz, J. Am. Chem. Soc., 2021, 143, 9961 66. S. Geiger, O. Kasian, M. Ledendecker, E. Pizzutilo, A. M. Mingers, W. T. Fu, O. Diaz-Morales, Z. Li, T. Oellers, L. Fruchter, A. Ludwig, K. J. J. Mayrhofer, M. T. M. Koper, S. Cherevko, Nat. Catal., 2018, 1, 508 67. C. Yang and A. Grimaud, Catalysts, 2017, 7, 149 68. R. R. Rao, M. J. Kolb, N. B. Halck, A. F. Pedersen, A. Mehta, H. You, K. A. Stoerzinger, Z. Feng, H. A. Hansen, H. Zhou, L. Giordano, J. Rossmeisl, T. Vegge, I. Chorkendorff, I. E. L. Stephens, Y. Shao-Horn, Energy Environ. Sci., 2017, 10, 2626 69. K. Schweinar, B. Gault, I. Mouton, O. Kasian, J. Phys. Chem. Lett., 2020, 11, 5008 70. Y. Wang, R. Ma, Z. Shi, H. Wu, S. Hou, Y. Wang, C. Liu, J. Ge, W. Xing, Chem, 2023, 9, 2931 71. C. H. Choi, C. Baldizzone, J.-P. Grote, A. K. Schuppert, F. Jaouen, K. J. J. Mayrhofer, Angew. Chem. Int. Ed., 2015, 54, 12753 72. K. Beppu, K. Obigane, F. Amano, Catal. Sci. Technol., 2023, 13, 6653 73. M. Etzi Coller Pascuzzi, M. van Velzen, J. P. Hofmann, E. J. M. Hensen, ChemCatChem, 2021, 13, 459 74. J. D. Benck, B. A. Pinaud, Y. Gorlin, T. F. Jaramillo, PLoS One, 2014, 9, e107942 75. A. Angulo, P. van der Linde, H. Gardeniers, M. Modestino, D. Fernández Rivas, Joule, 2020, 4, 555 76. F. Struyven, M. Sellier, P. Mandin, Int. J. Hydrogen Energy, 2023, 48, 32607 77. M. Li, J. Wei, L. Ren, Y. Zhao, Z. Shang, D. Zhou, W. Liu, L. Luo, X. Sun, Cell Rep. Phys. Sci., 2021, 2, 100374 78. Z. Li, R. Hu, J. Song, L. Liu, J. Qu, W. Song, C. Cao, Adv. Mater. Interfaces, 2021, 8, 2001636 79. W. Xu, Z. Lu, X. Sun, L. Jiang, X. Duan, Acc. Chem. Res., 2018, 51, 1590 80. C. Song, D. Jin, S. Choi, Y. Lee, J. Mater. Chem. A, 2023, 11, 26626 81. M. Bernt, A. Hartig-Weiß, M. F. Tovini, H. A. El-Sayed, C. Schramm, J. Schröter, C. Gebauer, H. A. Gasteiger, Chem. Ing. Tech., 2020, 92, 31 82. H. Wang, Z.-n. Chen, Y. Wang, D. Wu, M. Cao, F. Sun, R. Cao, Natl. Sci. Rev., 2024, 11, nwae056 83. Z.-Y. Wu, F.-Y. Chen, B. Li, S.-W. Yu, Y. Z. Finfrock, D. M. Meira, Q.-Q. Yan, P. Zhu, M.-X. Chen, T.-W. Song, Z. Yin, H.-W. Liang, S. Zhang, G. Wang, H. Wang, Nat. Mater., 2023, 22, 100 84. G. Fan, F. Li, D. G. Evans, X. Duan, Chem. Soc. Rev., 2014, 43, 7040 85. Z. Shi, J. Li, Y. Wang, S. Liu, J. Zhu, J. Yang, X. Wang, J. Ni, Z. Jiang, L. Zhang, Y. Wang, C. Liu, W. Xing, J. Ge, Nat. Commun., 2023, 14, 843 86. J. Chen, Y. Ma, T. Huang, T. Jiang, S. Park, J. Xu, X. Wang, Q. Peng, S. Liu, G. Wang, W. Chen, Adv. Mater., 2024, 36, 2312369 87. S. Wang, J. Zang, W. Shi, D. Zhou, Y. Jia, J. Wu, W. Yan, B. Zhang, L. Sun, K. Fan, ACS Appl. Mater. Interfaces, 2023, 15, 59432 88. Y.-N. Zhou, N. Yu, Q.-X. Lv, B. Liu, B. Dong, Y.-M. Chai, J. Mater. Chem. A, 2022, 10, 16193 89. P. Strasser, Acc. Chem. Res., 2016, 49, 2658 90. S. Pan, H. Li, D. Liu, R. Huang, X. Pan, D. Ren, J. Li, M. Shakouri, Q. Zhang, M. Wang, C. Wei, L. Mai, B. Zhang, Y. Zhao, Z. Wang, M. Graetzel, X. Zhang, Nat. Commun., 2022, 13, 2294 91. S. Kong, A. Li, J. Long, K. Adachi, D. Hashizume, Q. Jiang, K. Fushimi, H. Ooka, J. Xiao, R. Nakamura, Nat. Catal., 2024, 7, 252 92. H. Zhao, L. Zhu, J. Yin, J. Jin, X. Du, L. Tan, Y. Peng, P. Xi, C.-H. Yan, Angew. Chem. Int. Ed., 2024, 63, e202402171 93. M. Lu, Y. Zheng, Y. Hu, B. Huang, D. Ji, M. Sun, J. Li, Y. Peng, R. Si, P. Xi, C.-H. Yan, Sci. Adv., 2022, 8, eabq3563 94. J. Hwang, R. R. Rao, L. Giordano, Y. Katayama, Y. Yu, Y. Shao-Horn, Science, 2017, 358, 751 95. S. Stølen, E. Bakken, C. E. Mohn, Phys. Chem. Chem. Phys., 2006, 8, 429 96. T. Bhowmik, M. K. Kundu, S. Barman, ACS Appl. Mater. Interfaces, 2016, 8, 28678 97. J. Chen, P. Cui, G. Zhao, K. Rui, M. Lao, Y. Chen, X. Zheng, Y. Jiang, H. Pan, S. X. Dou, W. Sun, Angew. Chem. Int. Ed., 2019, 58, 12540 98. X. Wang, W. Chen, L. Zhang, T. Yao, W. Liu, Y. Lin, H. Ju, J. Dong, L. Zheng, W. Yan, X. Zheng, Z. Li, X. Wang, J. Yang, D. He, Y. Wang, Z. Deng, Y. Wu, Y. Li, J. Am. Chem. Soc., 2017, 139, 9419 99. X. Bai, X. Zhang, Y. Sun, M. Huang, J. Fan, S. Xu, H. Li, Angew. Chem. Int. Ed., 2023, 62, e202308704 100. J. Zhang, D. Zhu, J. Yan, C.-A. Wang, Nat. Commun., 2021, 12, 6665 101. C. A. Campos-Roldán, R. G. González-Huerta, N. Alonso-Vante, Electrochim. Acta, 2018, 283, 1829 102. A. Mahdavi-Shakib, T. N. Whittaker, T. Y. Yun, K. B. Sravan Kumar, L. C. Rich, S. Wang, R. M. Rioux, L. C. Grabow, B. D. Chandler, Nat. Catal., 2023, 6, 710 103. X. Wang, X. Wan, X. Qin, C. Chen, X. Qian, Y. Guo, Q. Xu, W.-B. Cai, H. Yang, K. Jiang, ACS Catal., 2022, 12, 9437 104. J. Xia, R. Gao, Y. Yang, Z. Tao, Z. Han, S. Zhang, Y. Xing, P. Yang, X. Lu, G. Zhou, ACS Nano, 2022, 16, 19133 105. J. Dai, Y. Zhu, H. A. Tahini, Q. Lin, Y. Chen, D. Guan, C. Zhou, Z. Hu, H.-J. Lin, T.-S. Chan, C.-T. Chen, S. C. Smith, H. Wang, W. Zhou, Z. Shao, Nat. Commun., 2020, 11, 5657 106. B. Yang, Z. Zhang, P. Liu, X. Fu, J. Wang, Y. Cao, R. Tang, X. Du, W. Chen, S. Li, H. Yan, Z. Li, X. Zhao, G. Qin, X.-Q. Chen, L. Zuo, Nature, 2023, 622, 499 107. S. Zhao, S.-F. Hung, L. Deng, W.-J. Zeng, T. Xiao, S. Li, C.-H. Kuo, H.-Y. Chen, F. Hu, S. Peng, Nat. Commun., 2024, 15, 2728 108. I. A. Khan, P. Morgen, S. Gyergyek, R. Sharma, S. M. Andersen, Mater. Chem. Phys., 2023, 308, 128192 109. G.-F. Li, D. Yang, P.-Y. Abel Chuang, ACS Catal., 2018, 8, 11688 110. E. Passalacqua, F. Lufrano, G. Squadrito, A. Patti, L. Giorgi, Electrochim. Acta, 2001, 46, 799 111. J. Wei, Y. Wang, C. Ke, Y. Liu, S. Yang, M. Pan, G. Li, Int. J. Hydrogen Energy, 2024, 69, 1539 112. C. N. Lunardi, A. J. Gomes, F. S. Rocha, J. De Tommaso, G. S. Patience, Can. J. Chem. Eng., 2021, 99, 627 113. H. Wang, Z. Xu, W. Lin, X. Yang, X. Gu, W. Zhu, Z. Zhuang, Nano Res., 2023, 16, 420 114. V. Karimi, R. Sharma, P. Morgen, S. M. Andersen, ACS Appl. Mater. Interfaces, 2023, 15, 49233 115. L. Zhou, Y. Shao, F. Yin, J. Li, F. Kang, R. Lv, Nat. Commun., 2023, 14, 7644 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Schematic illustration of the degradation mechanisms of oxygen evolution reaction (OER) catalysts under acidic conditions.
-
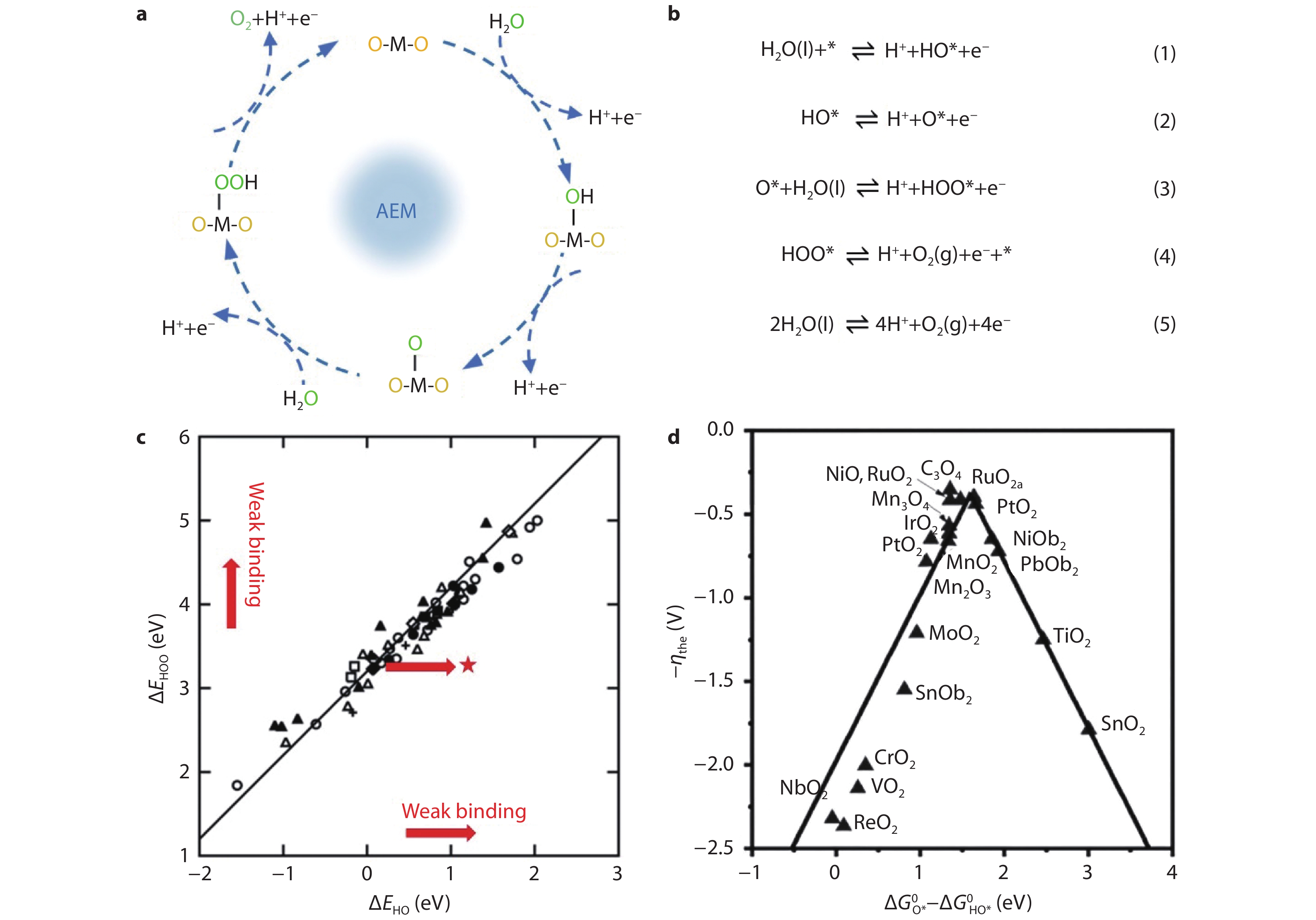
Figure 1.
Adsorbate evolution mechanism (AEM) for oxygen evolution reaction (OER). a Schematic illustration and b Specific reaction steps of AEM pathway for OER under acidic conditions. c Correlation between the binding energies of HO* and HOO*. d Theoretical overpotential for the OER as the function of the Gibbs free energy change of HO* and O* (ΔGO* − ΔGHO*).[16] Copyright 2011, Wiley-VCH Verlag GmbH.
-
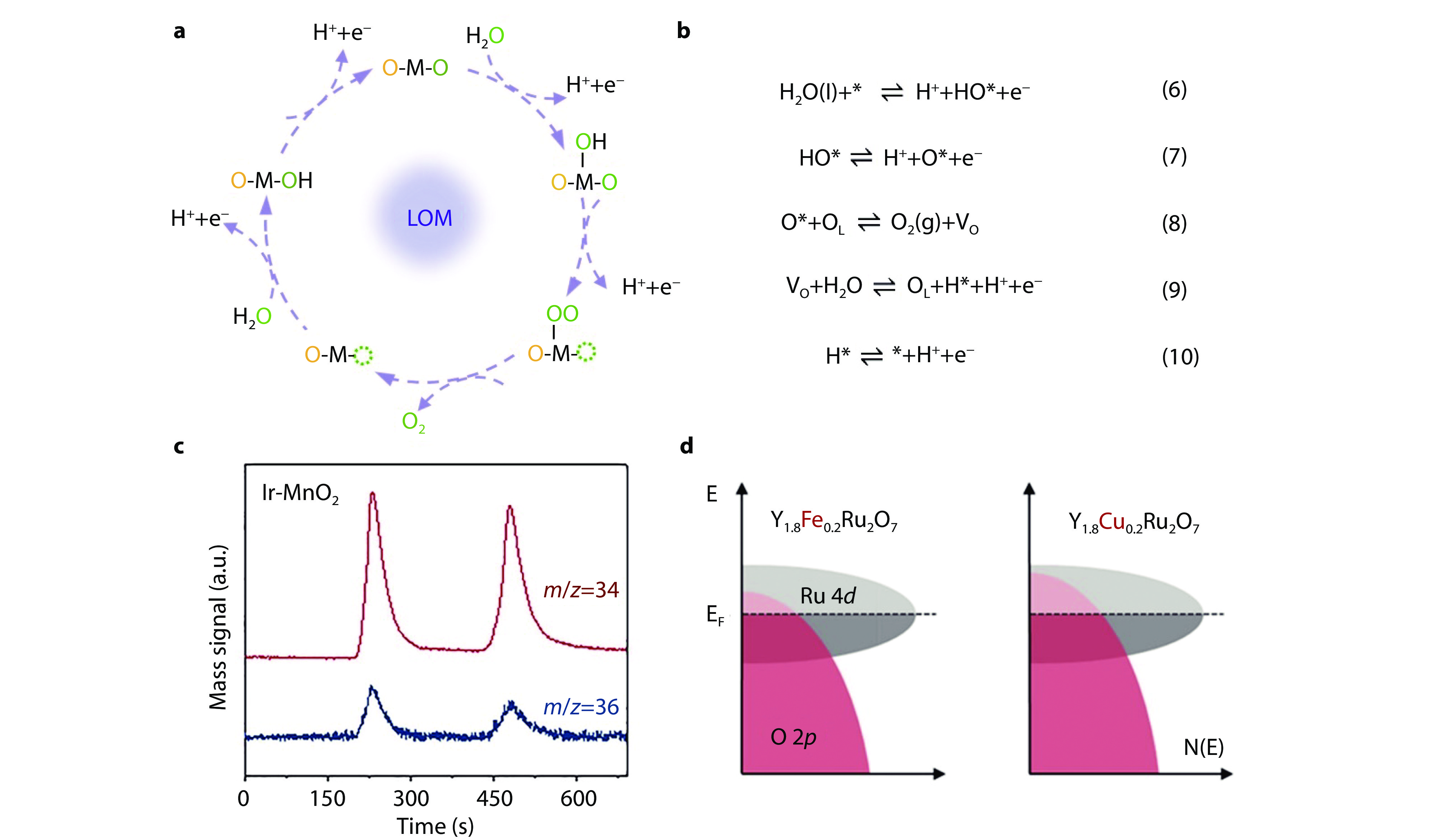
Figure 2.
Lattice oxygen oxidation mechanism (LOM) and experimental insights into oxygen participation. a Schematic illustration and b Specific reaction steps of LOM pathway for OER under acidic conditions. c Differential electrochemical mass spectrometry (DEMS) analysis of 34O2 and 36O2 signals from reaction products for 18O-labeled Ir-MnO2 in 0.5 M H2SO4 using H216O.[43] Copyright 2021, Elsevier. d Scheme of band diagrams for Ru-O covalent bond of Y1.8Fe0.2Ru2O7 and Y1.8Cu0.2Ru2O7.[44] Copyright 2020, American Chemical Society.
-
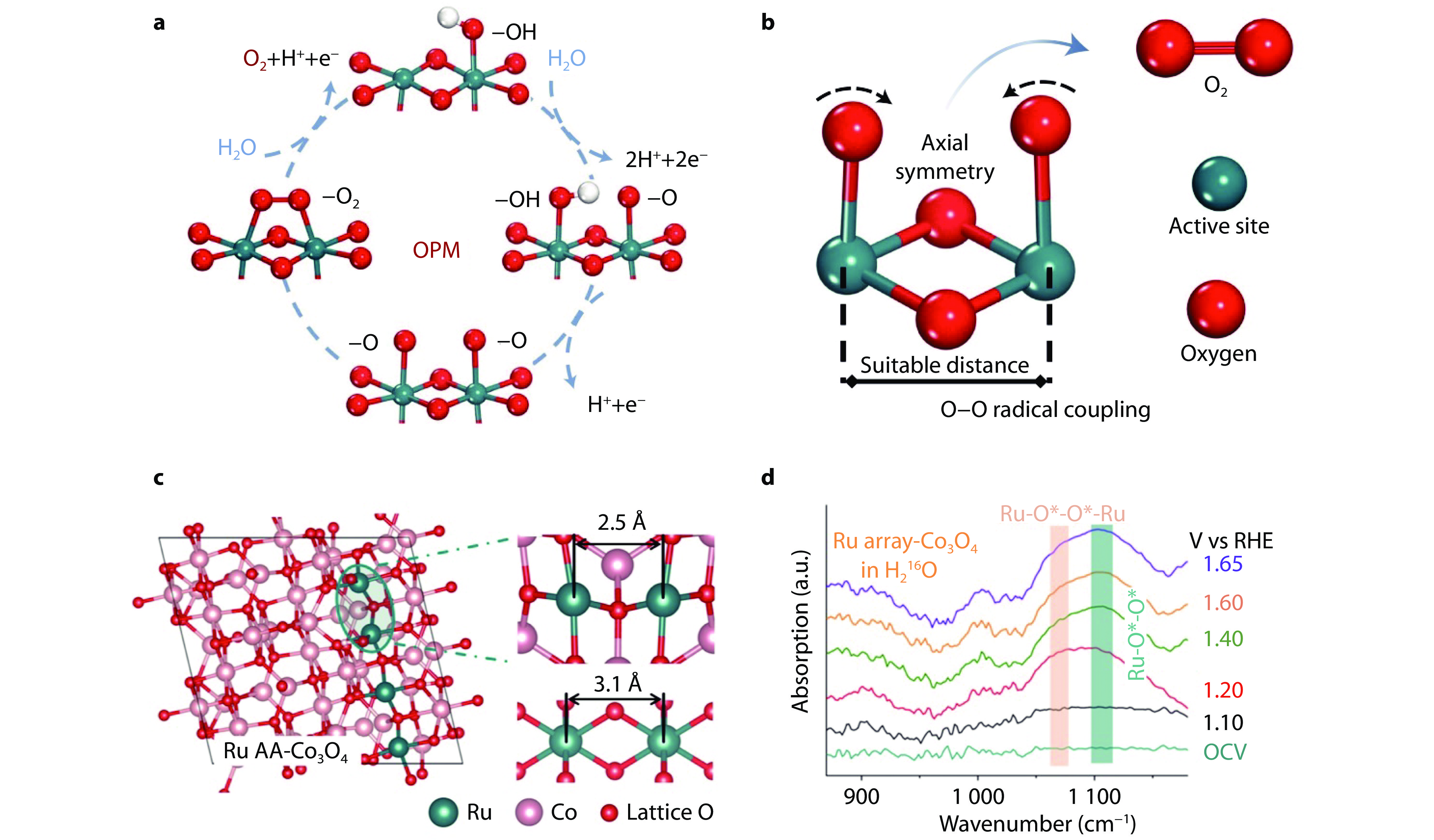
Figure 3.
Oxide path mechanism (OPM) for oxygen evolution and catalytic enhancement through dual active sites. a Schematic illustration of OPM pathway. b O-O radical coupling facilitated by symmetrical dual active sites.[48] Copyright 2021, Nature Publishing Group. c Schematic illustration of Ru atom array-doped Co3O4 (Ru AA-Co3O4). d Operando attenuated total reflectance surface-enhanced IR spectroscopy (ATR-SEIRAS) spectra of 18O-labeled Ru AA-Co3O4 in H216O electrolyte.[49] Copyright 2024, American Chemical Society.
-
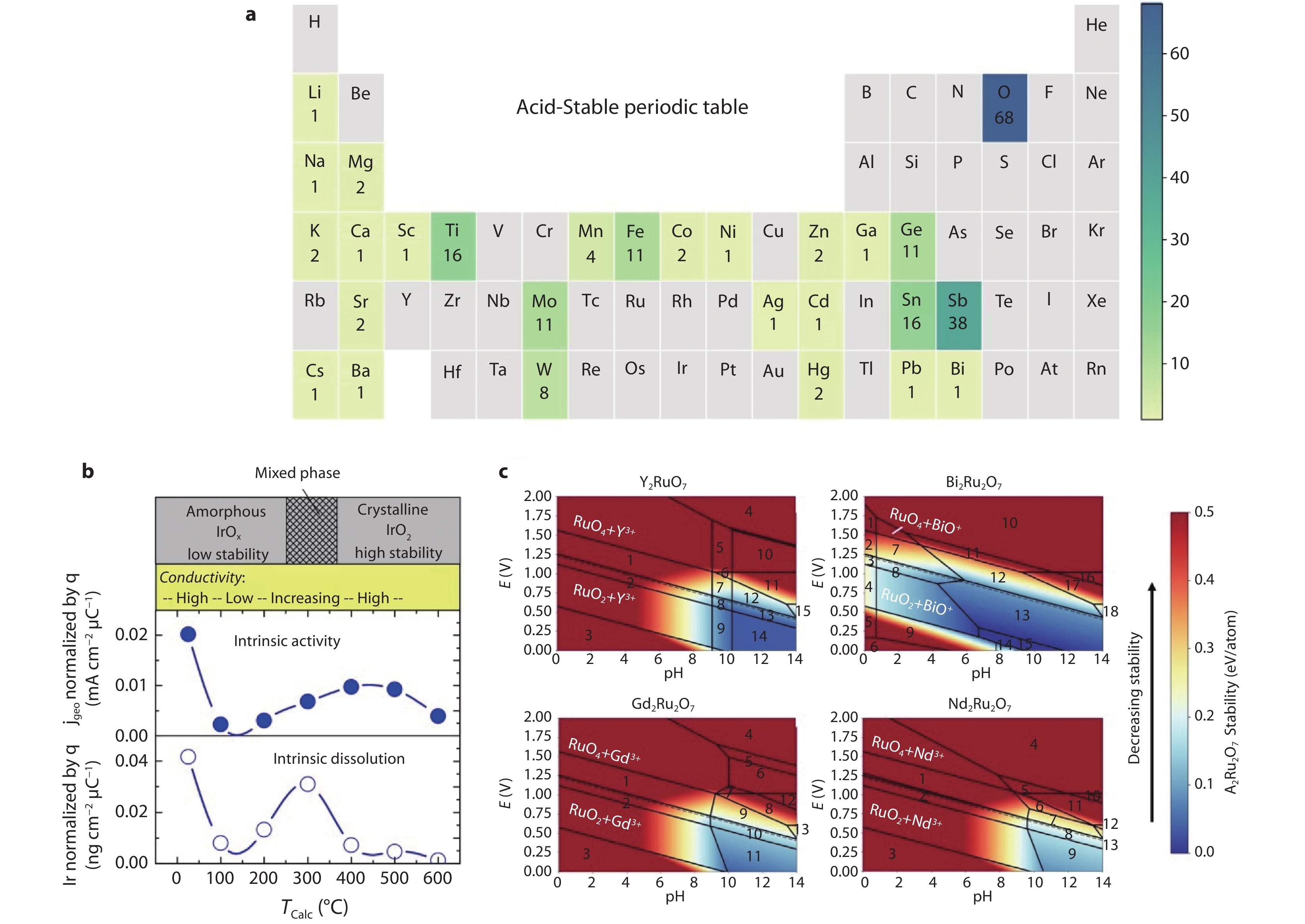
Figure 4.
Elemental composition and stability of OER catalysts under acidic conditions. a Frequency at which each element appears in acid-stable oxides. Elements with zero frequency are shaded in gray. Sb/Ti/Sn/Ge/Mo/W-based oxides tend to remain stable in strong acids.[57] Copyright 2020, American Chemical Society. b Correlation between calcination temperature and the crystallinity and stability of IrOx/Ti.[59] Copyright 2016, The Electrochemical Society. c Pourbaix diagrams of Ru-based perovskites calculated to evaluate their thermodynamic stability.[60] Copyright 2020, American Chemical Society.
-
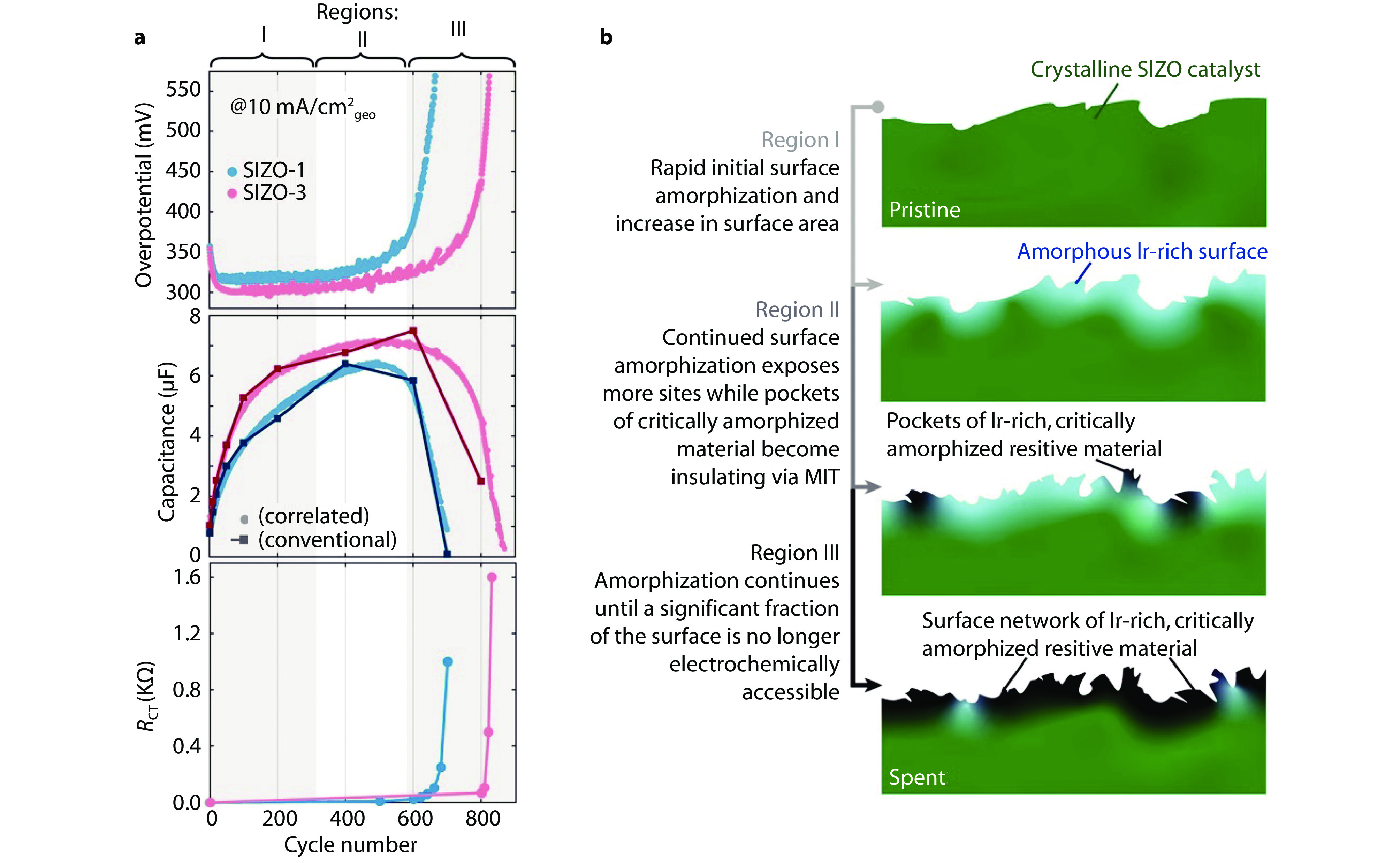
Figure 5.
Structural changes and degradation of OER catalysts during prolonged cycling. a Evolution of overpotential at 10 mA cm−2geo (current density normalized by geometric area), electrochemical capacitance, charge transfer resistance (RCT) of the electrode in Regions I, II, and III during prolonged cycling stability assessments. b Graphical depiction of the surface amorphization and metal to insulator transition (MIT) processes in the SrIr0.8Zn0.2O3 (SIZO) catalyst during prolonged electrochemical cycling.[65] Copyright 2021, American Chemical Society.
-
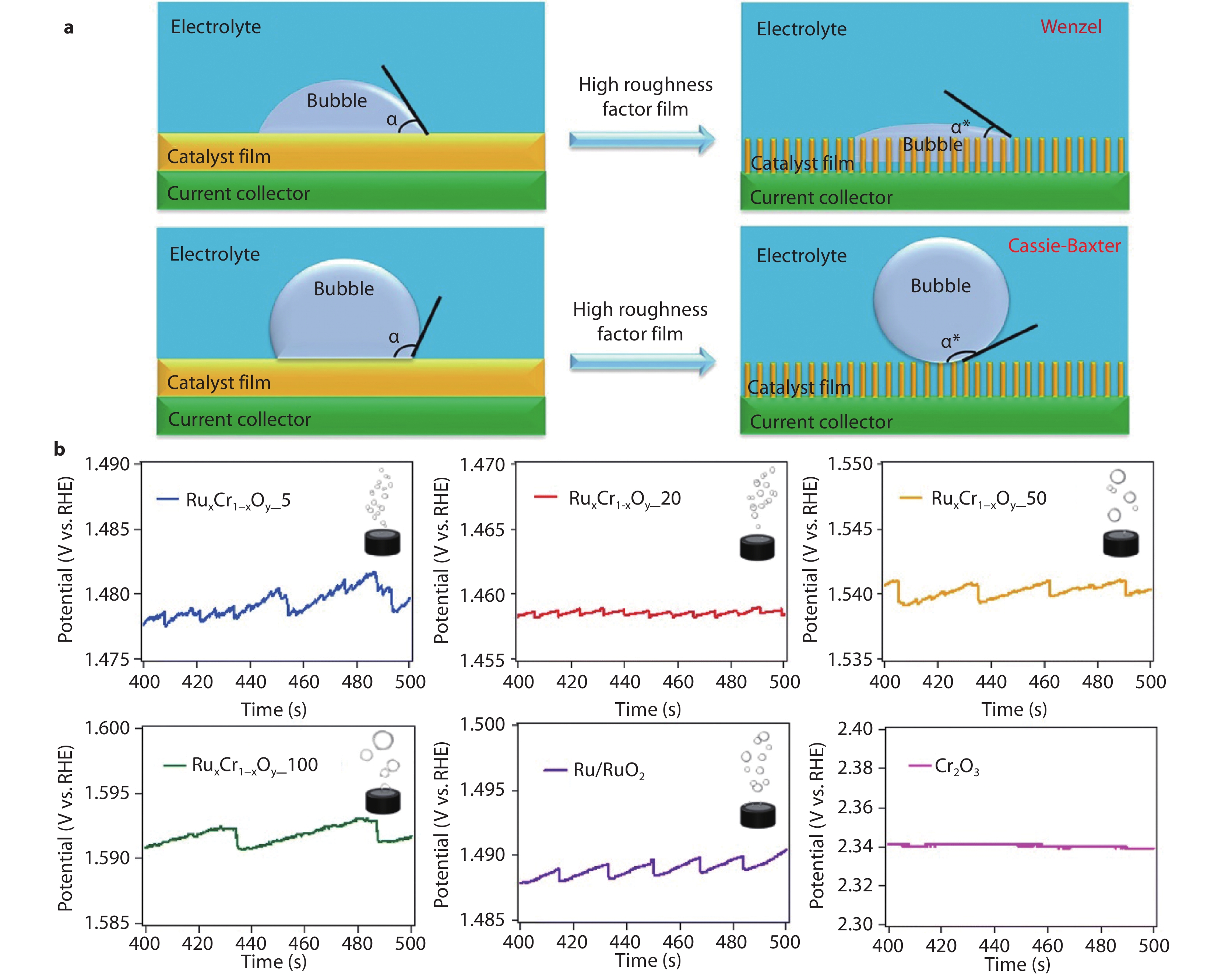
Figure 6.
Influence of surface roughness and catalyst morphology on bubble detachment efficiency. a Schematic illustration of how the surface roughness affecting the bubble contact angle at given intrinsic aerophilicity.[79] Copyright 2018, American Chemical Society. b Chronopotentiometric monitoring of generated O2 gas desorption for different samples. Potential changes were measured at a constant current density of 5 mA cm−2 between 400 and 500 s. The insets of the respective chronopotentiograms present a schematic illustration of the O2 gas desorption behaviour for the corresponding electrode catalysts.[80] Copyright 2023, The Royal Society of Chemistry.
-
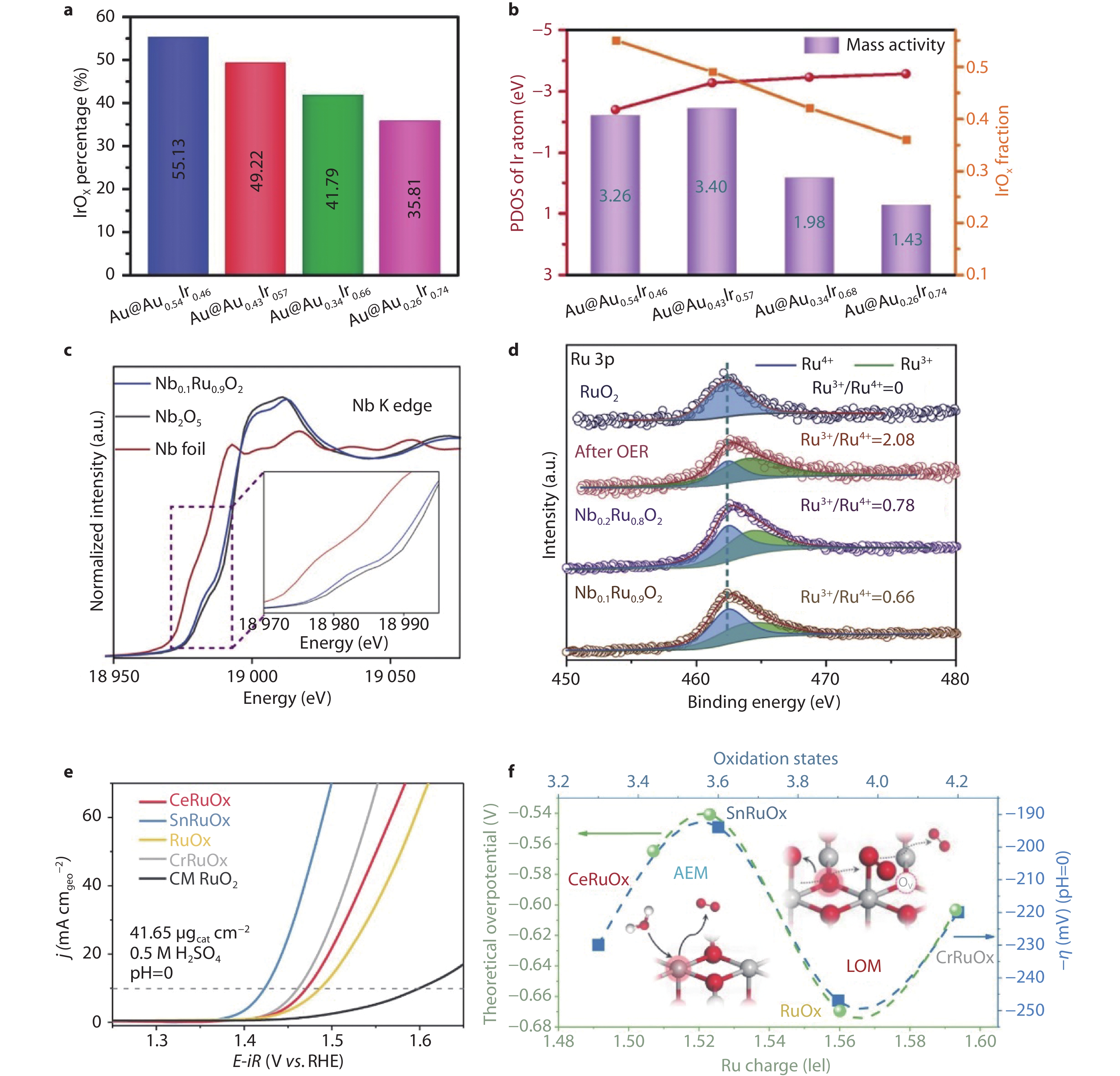
Figure 7.
Strategies to enhance OER stability and activity through structural modifications. a IrOx percentage (estimated by EXAFS analysis) for Au@AuxIr1−x. b Structure–activity relationships.[82] Copyright 2024, Oxford University Press. c Nb K-edge spectra of Nb foil, Nb0.1Ru0.9O2, and Nb2O5. Inset is the enlarged image of selected area. d Ru 3p XPS spectra of Nb0.1Ru0.9O2, Nb0.2Ru0.8O2, and Nb0.1Ru0.9O2 after stability test and pure RuO2.[12] Copyright 2023, Elsevier. e Normalized LSV curves of MRuOx. f The variation of apparent overpotential at 10 mA cm−2 with Ru oxidation states.[85] Copyright 2023, Springer Nature.
-
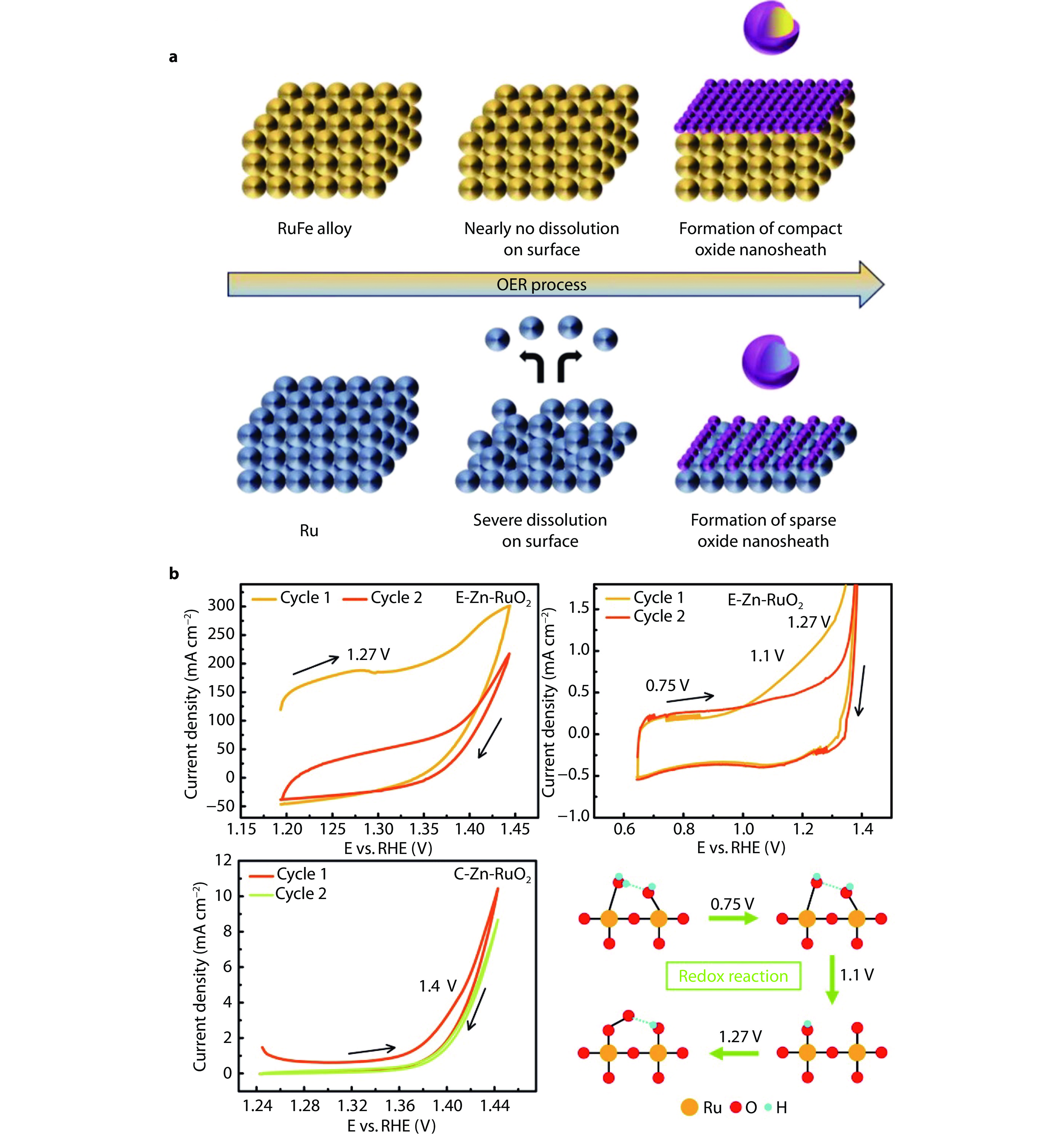
Figure 8.
Surface reconstruction and its impact on catalyst stability. a Schematic of surface reconstruction for RuFe@CF and Ru@CF during OER process in acidic media.[86] Copyright 2024, Wiley-VCH GmbH. b Pseudocapacitive behaviour in the first and second cycles during CV of E–Zn–RuO2 and C–Zn–RuO2, and the redox reaction on Ru sites during the acidic OER process.[88] Copyright 2022, Royal Society of Chemistry.
-
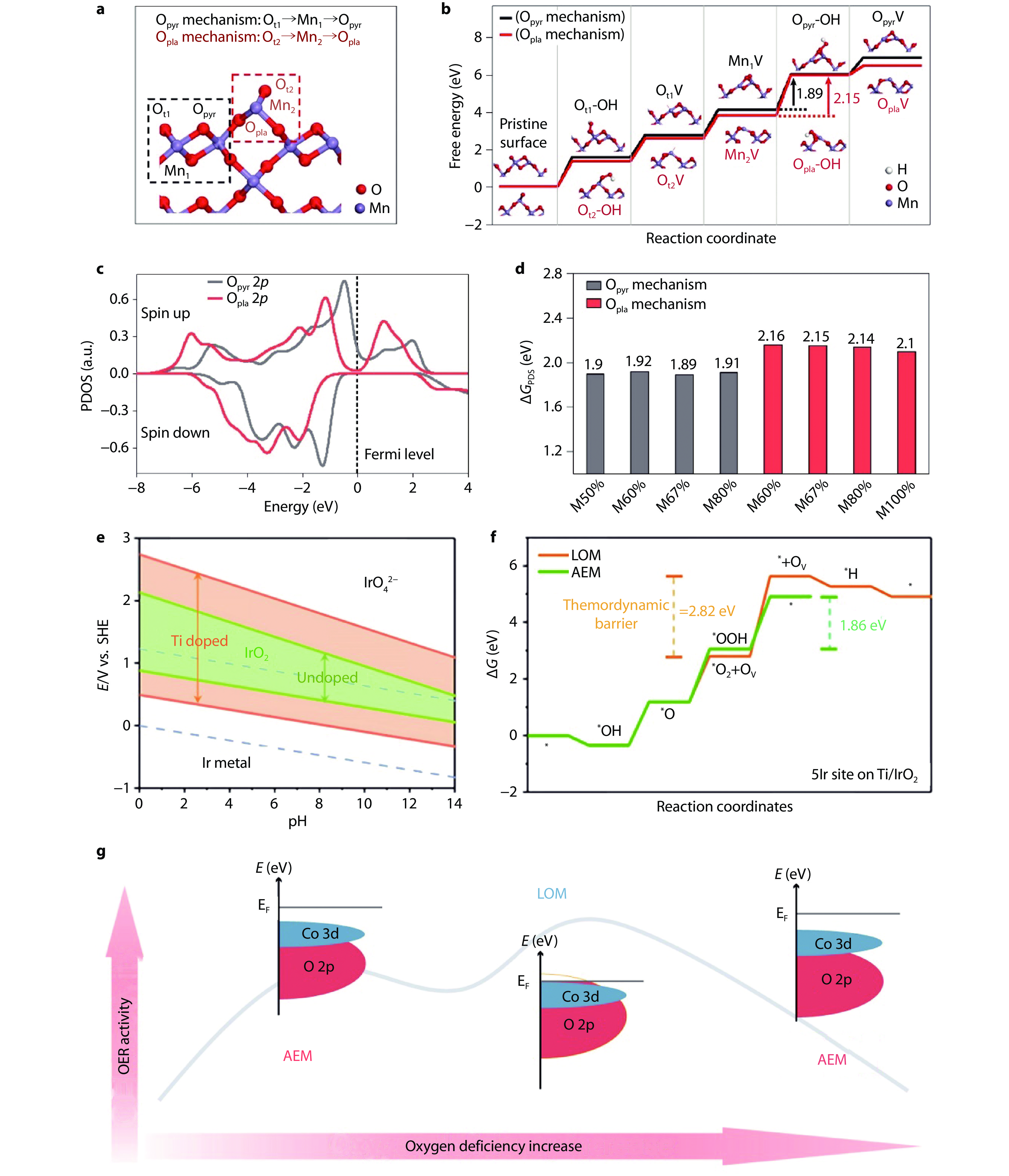
Figure 9.
Role of structural parameters and oxygen vacancies in enhancing catalyst stability. a Atomic structure of catalyst with 67% planar oxygen (Opla) concentrations (M67%) and the sites involved in the Opla and pyramidal oxygen (Opyr) mechanisms are indicated in red and black boxes, respectively. Ot1 and Ot2 represent terminal oxygens. b Free-energy diagrams for the two dissolution mechanisms on the (001) surface of M67%. c Projected density of states (PDOS) for the 2p orbitals of Opyr and Opla at the M67% (001) surface. d Calculated ΔGPDS of the Opyr and Opla mechanisms for dissolution of MnO2 with different Opla concentrations.[91] Copyright 2024, Springer Nature. e Pourbaix diagrams of 4 atoms’ model with and without Ti doping calculated by DFT. f OER free energy diagrams at the 5Ir site of Ti-IrO2 surface based on AEM and LOM.[70] Copyright 2023, Elsevier. g Illustration of mechanism shift and electronic structure-stability relationship of OER controlled by oxygen defect contents.[93] Copyright 2022, American Association for the Advancement of Science.
-
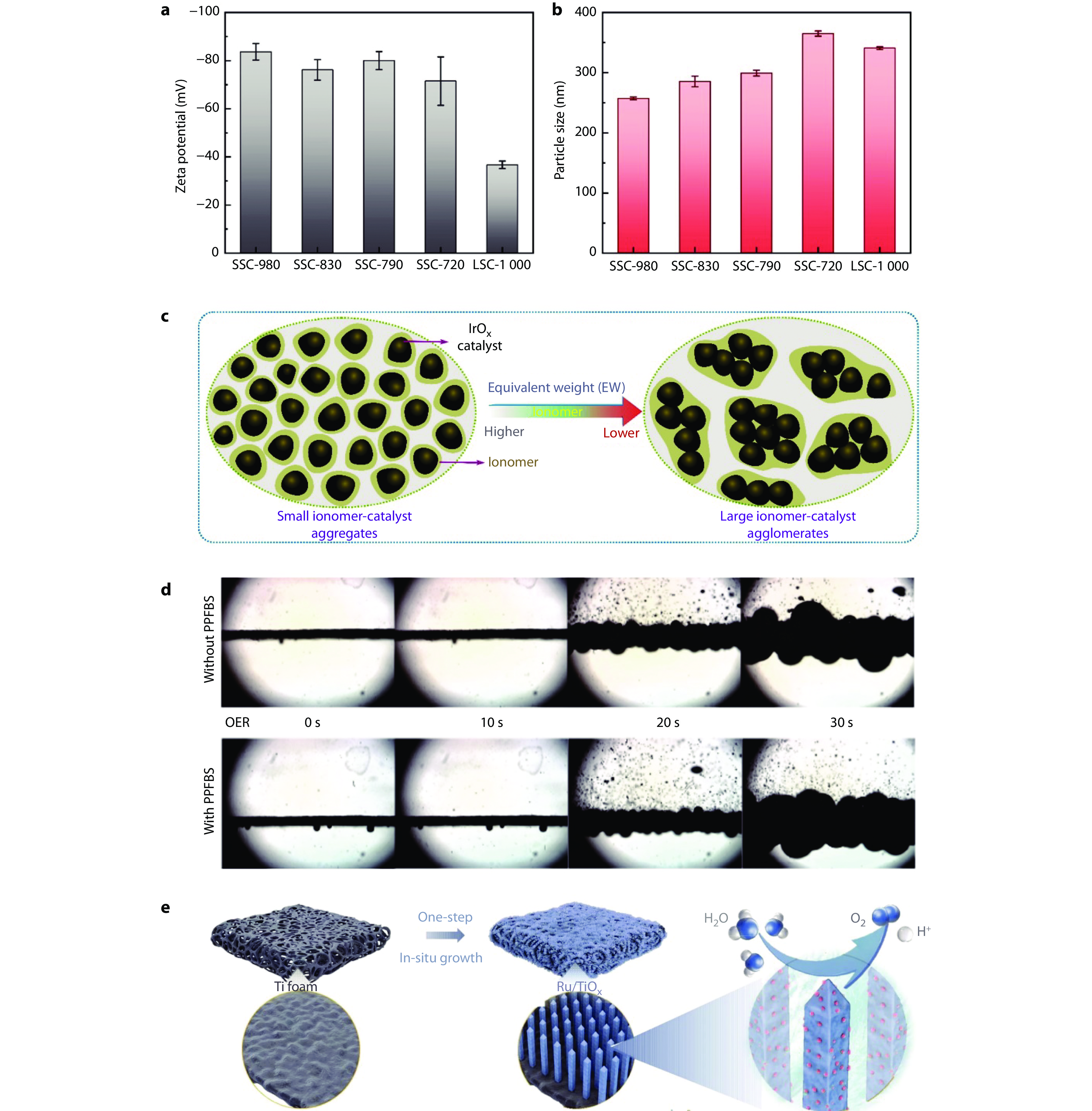
Figure 10.
Optimizing catalyst-ionomer interactions for enhanced OER performance and stability. a Zeta potential and b particle size of catalyst inks. Error bars are standard deviations from three independent measurements. c Schematic illustration to elucidate ionomer equivalent weight (EW) effect on the catalyst layer (CL) structure.[111] Copyright 2024, Elsevier. d Microscope images of bubble formation on catalyst-supported carbon papers at different elapsed times of the chronoamperometry tests in 0.5M H2SO4 with or without potassium perfluorobutyl sulfonate (PPFBS).[113] Copyright 2022, Springer Nature. e Schematic illustration of synthesis of Ru/TiOx as binder-free electrode towards oxygen evolution reaction (OER) in acidic media.[115] Copyright 2023, Springer Nature.

 Yujia Long is currently a Ph.D. student at the School of Materials Science and Engineering, Tsinghua University, under the supervision of Prof. Ruitao Lv since 2023. Her research interests include the rational design and synthesis of efficient electrocatalysts for oxygen evolution reaction, etc.
Yujia Long is currently a Ph.D. student at the School of Materials Science and Engineering, Tsinghua University, under the supervision of Prof. Ruitao Lv since 2023. Her research interests include the rational design and synthesis of efficient electrocatalysts for oxygen evolution reaction, etc.  Ruitao Lv is a professor at the School of Materials Science and Engineering, Tsinghua University. He received his Ph.D. degree in 2009 from Tsinghua University. He has been a visiting researcher at Kyushu University (Japan) and a postdoctoral researcher at Penn State University (USA). He joined Tsinghua University as a faculty member in 2013. His research interests include the defect engineering of 2D layered materials and their applications in electrocatalysis, molecular sensing, optoelectronic devices, etc.
Ruitao Lv is a professor at the School of Materials Science and Engineering, Tsinghua University. He received his Ph.D. degree in 2009 from Tsinghua University. He has been a visiting researcher at Kyushu University (Japan) and a postdoctoral researcher at Penn State University (USA). He joined Tsinghua University as a faculty member in 2013. His research interests include the defect engineering of 2D layered materials and their applications in electrocatalysis, molecular sensing, optoelectronic devices, etc. 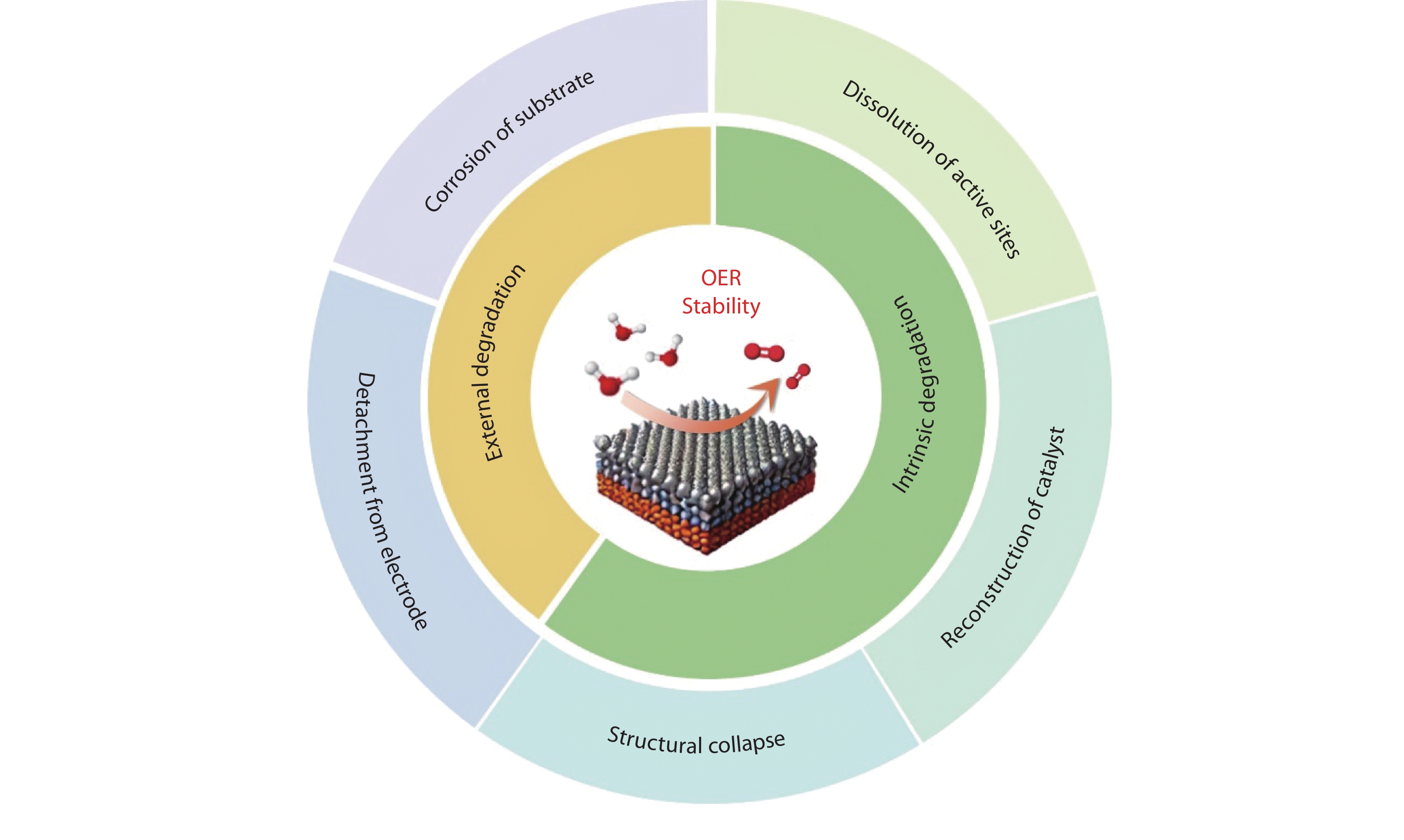

 DownLoad:
DownLoad: