| Citation: | Yue Yang, Jianghao Wang, Zhichong Feng, Xiaotong Li, Hao Bin Wu. Catalyst design for CO2 electroreduction under acidic conditions[J]. Energy Lab, 2025, 3(3): 250006. doi: 10.54227/elab.20250006 |
Catalyst design for CO2 electroreduction under acidic conditions
-
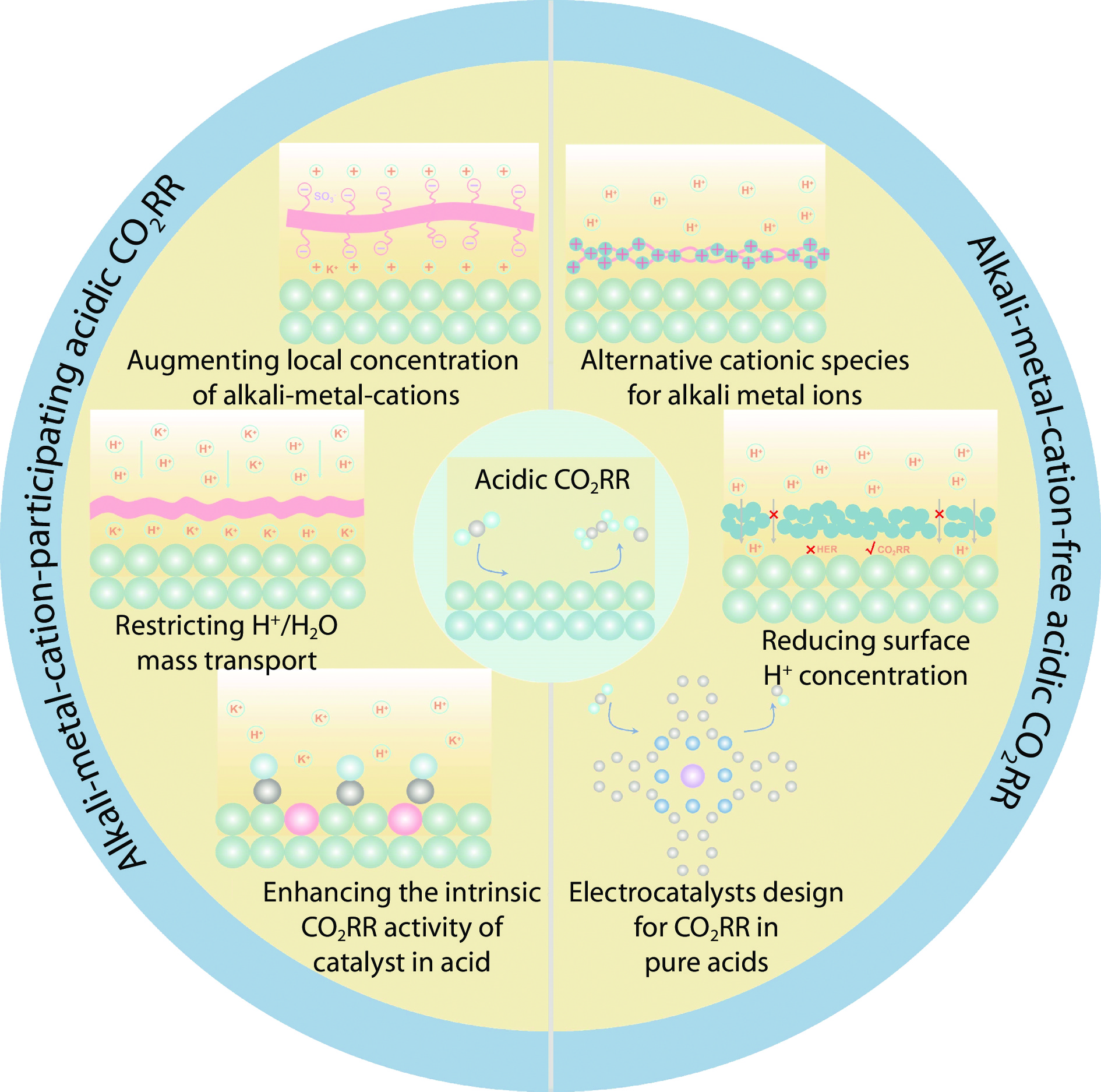
Abstract
CO2 reduction under acidic conditions has progressively gained attention due to advancements in carbon efficiency. However, acidic conditions present significant challenges for CO2 reduction, including intensified hydrogen evolution and hampered carbon-carbon coupling. In this review, we examine a broad spectrum of recently developed catalyst design strategies aiming at achieving effective CO2 reduction under acidic conditions. Strategies such as augmenting local cation concentration, restricting H+/H2O transport and enhancing intrinsic catalytic activity suppress hydrogen evolution and facilitate CO2 reduction in alkali-metal-cation-containing systems. Moreover, seeking alternative organic cations to assist CO2RR, reducing surface H+ concentration and developing electrocatalysts operated in pure acids would address the drawbacks associated with alkali metal cations. Finally, we discuss potential future directions for CO2 reduction under acidic conditions.
-
-
References
1. S. M. Jordaan, C. Wang, Nat. Catal., 2021, 4, 915 2. P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo, E. H. Sargent, Science, 2019, 364, 350 3. M. B. Ross, P. De Luna, Y. Li, C. -T. Dinh, D. Kim, P. Yang, E. H. Sargent, Nat. Catal., 2019, 2, 648 4. Y. Zhao, L. Hao, A. Ozden, S. J. Liu, R. K. Miao, P. F. Ou, T. Alkayyali, S. Z. Zhang, J. Ning, Y. X. Liang, Y. Xu, M. Y. Fan, Y. J. Chen, J. E. Huang, K. Xie, J. Q. Zhang, C. P. O'Brien, F. W. Li, E. H. Sargent, D. Sinton, Nat. Synth., 2023, 2, 403 5. C. T. Dinh, T. Burdyny, M. G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. P. G. de Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Q. Zou, R. Quintero-Bermudez, Y. J. Pang, D. Sinton, E. H. Sargent, Science, 2018, 360, 783 6. Z. Wang, Y. Li, X. Zhao, S. Chen, Q. Nian, X. Luo, J. Fan, D. Ruan, B. -Q. Xiong, X. Ren, J. Am. Chem. Soc., 2023, 145, 6339 7. M. Zhong, K. Tran, Y. Min, C. Wang, Z. Wang, C. -T. Dinh, P. De Luna, Z. Yu, A. S. Rasouli, P. Brodersen, S. Sun, O. Voznyy, C. -S. Tan, M. Askerka, F. Che, M. Liu, A. Seifitokaldani, Y. Pang, S. -C. Lo, A. Ip, Z. Ulissi, E. H. Sargent, Nature, 2020, 581, 178 8. S. Li, X. Dong, Y. Zhao, J. Mao, W. Chen, A. Chen, Y. Song, G. Li, Z. Jiang, W. Wei, Y. Sun, Angew. Chem. Int. Ed., 2022, 61, e202210432 9. M. Zheng, P. T. Wang, X. Zhi, K. Yang, Y. Jiao, J. J. Duan, Y. Zheng, S. Z. Qiao, J. Am. Chem. Soc., 2022, 144, 14936 10. Z. Z. Niu, L. P. Chi, R. Liu, Z. Chen, M. R. Gao, Energy Environ. Sci., 2021, 14, 4169 11. C. P. O’Brien, R. K. Miao, S. Liu, Y. Xu, G. Lee, A. Robb, J. E. Huang, K. Xie, K. Bertens, C. M. Gabardo, J. P. Edwards, C. -T. Dinh, E. H. Sargent, D. Sinton, ACS Energy Lett., 2021, 6, 2952 12. A. Ozden, F. P. G. De Arquer, J. E. Huang, J. Wicks, J. Sisler, R. K. Miao, C. P. O'Brien, G. Lee, X. Wang, A. H. Ip, E. H. Sargent, D. Sinton, Nat. Sustainability, 2022, 5, 563 13. J. A. Rabinowitz, M. W. Kanan, Nat. Commun., 2020, 11, 5231 14. Y. Chen, X. Y. Li, Z. Chen, A. Ozden, J. E. Huang, P. Ou, J. Dong, J. Zhang, C. Tian, B. H. Lee, X. Wang, S. Liu, Q. Qu, S. Wang, Y. Xu, R. K. Miao, Y. Zhao, Y. Liu, C. Qiu, J. Abed, H. Liu, H. Shin, D. Wang, Y. Li, D. Sinton, E. H. Sargent, Nat. Nanotechnol., 2024, 19, 311 15. J. Y. T. Kim, P. Zhu, F. -Y. Chen, Z. -Y. Wu, D. A. Cullen, H. Wang, Nat. Catal., 2022, 5, 288 16. M. Fan, J. E. Huang, R. K. Miao, Y. Mao, P. Ou, F. Li, X. -Y. Li, Y. Cao, Z. Zhang, J. Zhang, Y. Yan, A. Ozden, W. Ni, Y. Wang, Y. Zhao, Z. Chen, B. Khatir, C. P. O’Brien, Y. Xu, Y. C. Xiao, G. I. N. Waterhouse, K. Golovin, Z. Wang, E. H. Sargent, D. Sinton, Nat. Catal., 2023, 6, 763 17. W. Fang, W. Guo, R. Lu, Y. Yan, X. Liu, D. Wu, F. M. Li, Y. Zhou, C. He, C. Xia, H. Niu, S. Wang, Y. Liu, Y. Mao, C. Zhang, B. You, Y. Pang, L. Duan, X. Yang, F. Song, T. Zhai, G. Wang, X. Guo, B. Tan, T. Yao, Z. Wang, B. Y. Xia, Nature, 2024, 626, 86 18. J. E. Huang, F. W. Li, A. Ozden, A. S. Rasouli, F. P. G. de Arquer, S. J. Liu, S. Z. Zhang, M. C. Luo, X. Wang, Y. W. Lum, Y. Xu, K. Bertens, R. K. Miao, C. T. Dinh, D. Sinton, E. H. Sargent, Science, 2021, 372, 1074 19. M. C. O. Monteiro, M. F. Philips, K. J. P. Schouten, M. T. M. Koper, Nat. Commun., 2021, 12, 4943 20. M. C. O. Monteiro, F. Dattila, B. Hagedoorn, R. García-Muelas, N. López, M. T. M. Koper, Nat. Catal., 2021, 4, 654 21. J. Gu, S. Liu, W. Ni, W. Ren, S. Haussener, X. Hu, Nat. Catal., 2022, 5, 268 22. H. Li, H. Li, P. Wei, Y. Wang, Y. Zang, D. Gao, G. Wang, X. Bao, Energy Environ. Sci., 2023, 16, 1502 23. B. Pan, J. Fan, J. Zhang, Y. Luo, C. Shen, C. Wang, Y. Wang, Y. Li, ACS Energy Lett., 2022, 7, 4224 24. H. -G. Qin, Y. -F. Du, Y. -Y. Bai, F. -Z. Li, X. Yue, H. Wang, J. -Z. Peng, J. Gu, Nat. Commun., 2023, 14, 5640 25. Z. Xu, Y. Xie, Y. Wang, Materials Reports: Energy, 2023, 3, 100173 26. Y. Xie, P. Ou, X. Wang, Z. Xu, Y. C. Li, Z. Wang, J. E. Huang, J. Wicks, C. McCallum, N. Wang, Y. Wang, T. Chen, B. T. W. Lo, D. Sinton, J. C. Yu, Y. Wang, E. H. Sargent, Nat. Catal., 2022, 5, 564 27. Y. Cao, Z. Chen, P. Li, A. Ozden, P. Ou, W. Ni, J. Abed, E. Shirzadi, J. Zhang, D. Sinton, J. Ge, E. H. Sargent, Nat. Commun., 2023, 14, 2387 28. M. S. E. Houache, R. Safari, U. O. Nwabara, T. Rafaïdeen, G. A. Botton, P. J. A. Kenis, S. Baranton, C. Coutanceau, E. A. Baranova, ACS Appl. Energy Mater., 2020, 3, 8725 29. G. Lee, Y. C. Li, J. -Y. Kim, T. Peng, D. -H. Nam, A. Sedighian Rasouli, F. Li, M. Luo, A. H. Ip, Y. -C. Joo, E. H. Sargent, Nat. Energy, 2021, 6, 46 30. K. Yang, R. Kas, W. A. Smith, J. Am. Chem. Soc., 2019, 141, 15891 31. W. -G. Cui, F. Gao, G. Na, X. Wang, Z. Li, Y. Yang, Z. Niu, Y. Qu, D. Wang, H. Pan, Chem. Soc. Rev., 2024, 53, 10253 32. H. Ooka, M. C. Figueiredo, M. T. M. Koper, Langmuir, 2017, 33, 9307 33. X. Hai, W. A. Goddard, C. Tao, Y. Liu, Proc. Natl. Acad. Sci. U.S.A., 2017, 114, 201702405 34. W. Ma, S. Xie, T. Liu, Q. Fan, J. Ye, F. Sun, Z. Jiang, Q. Zhang, J. Cheng, Y. Wang, Nat. Catal., 2020, 3, 478 35. L. Li, Z. Liu, X. Yu, M. Zhong, Angew. Chem. Int. Ed., 2023, 62, e202300226 36. S. Feng, X. Wang, D. Cheng, Y. Luo, M. Shen, J. Wang, W. Zhao, S. Fang, H. Zheng, L. Ji, X. Zhang, W. Xu, Y. Liang, P. Sautet, J. Zhu, Angew. Chem. Int. Ed., 2024, 1, e202317942 37. B. Zhang, J. Zou, Z. Chen, W. Yan, W. Liu, C. Dong, D. Cai, Q. Zhang, Y. Wang, S. Xie, Next Nanotechnol., 2023, 2, 100014 38. Z. Li, X. Li, R. Wang, A. Campos Mata, C. S. Gerke, S. Xiang, A. Mathur, L. Zhang, D. -Z. Lin, T. Li, K. N. Jayarapu, A. Liu, L. Gupta, A. I. Frenkel, V. S. Thoi, P. M. Ajayan, S. Roy, Y. Liu, Y. Liu, Nat. Commun., 2025, 16, 3206 39. J. Fan, B. Pan, J. Wu, C. Shao, Z. Wen, Y. Yan, Y. Wang, Y. Li, Angew. Chem. Int. Ed., 2024, 1, e202317828 40. K. Xie, R. K. Miao, A. Ozden, S. Liu, Z. Chen, C. -T. Dinh, J. E. Huang, Q. Xu, C. M. Gabardo, G. Lee, J. P. Edwards, C. P. O’Brien, S. W. Boettcher, D. Sinton, E. H. Sargent, Nat. Commun., 2022, 13, 3609 41. B. Pan, Y. Wang, Y. Li, Chem Catal., 2022, 2, 1267 42. H. -G. Qin, F. -Z. Li, Y. -F. Du, L. -F. Yang, H. Wang, Y. -Y. Bai, M. Lin, J. Gu, ACS Catal., 2023, 13, 916 43. M. H. Hicks, W. Nie, A. E. Boehme, H. A. Atwater, T. Agapie, J. C. Peters, J. Am. Chem. Soc., 2024, 146, 25282 44. Y. Qin, C. Xia, T. Wu, J. Zhang, G. Gao, B. Y. Xia, M. L. Coote, S. Ding, Y. Su, J Am Chem Soc, 2024, 146, 32539 45. Z. -M. Zhang, T. Wang, Y. -C. Cai, X. -Y. Li, J. -Y. Ye, Y. Zhou, N. Tian, Z. -Y. Zhou, S. -G. Sun, Nat. Catal., 2024, 7, 807 46. S. Ringe, C. G. Morales-Guio, L. D. Chen, M. Fields, T. F. Jaramillo, C. Hahn, K. Chan, Nat. Commun., 2020, 11, 33 47. Z. Ma, Z. Yang, W. Lai, Q. Wang, Y. Qiao, H. Tao, C. Lian, M. Liu, C. Ma, A. Pan, H. Huang, Nat. Commun., 2022, 13, 7596 48. Z. Jiang, Z. Zhang, H. Li, Y. Tang, Y. Yuan, J. Zao, H. Zheng, Y. Liang, Adv. Energy Mater., 2022, 1, 2203603 49. Y. Qiao, W. Lai, K. Huang, T. Yu, Q. Wang, L. Gao, Z. Yang, Z. Ma, T. Sun, M. Liu, C. Lian, H. Huang, ACS Catal., 2022, 1, 2357 50. L. -P. Chi, Z. -Z. Niu, Y. -C. Zhang, X. -L. Zhang, J. Liao, Z. -Z. Wu, P. -C. Yu, M. -H. Fan, K. -B. Tang, M. -R. Gao, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2312876120 51. M. Liu, Y. J. Pang, B. Zhang, P. De Luna, O. Voznyy, J. X. Xu, X. L. Zheng, C. T. Dinh, F. J. Fan, C. H. Cao, F. P. G. de Arquer, T. S. Safaei, A. Mepham, A. Klinkova, E. Kumacheva, T. Filleter, D. Sinton, S. O. Kelley, E. H. Sargent, Nature, 2016, 537, 382 52. Y. -C. Li, X. -L. Zhang, X. -L. Tai, X. -P. Yang, P. -C. Yu, S. -C. Dong, L. -P. Chi, Z. -Z. Wu, Y. -C. Zhang, S. -P. Sun, P. -G. Lu, L. Zhu, F. -Y. Gao, Y. Lin, M. -R. Gao, Angew. Chem. Int. Ed., 2025, 1, e202422054 53. X. Zi, Y. Zhou, L. Zhu, Q. Chen, Y. Tan, X. Wang, M. Sayed, E. Pensa, R. A. Geioushy, K. Liu, J. Fu, E. Cortés, M. Liu, Angew. Chem. Int. Ed., 2023, 62, e202309351 54. Z. Wang, D. Liu, C. Xia, X. Shi, Y. Zhou, Q. Liu, J. Huang, H. Wu, D. Zhu, S. Zhang, J. Li, P. Deng, A. S. Vasenko, B. Y. Xia, X. Tian, Nat. Commun., 2025, 16, 1754 55. Q. Zhang, C. B. Musgrave, Y. Song, J. Su, L. Huang, L. Cheng, G. Li, Y. Liu, Y. Xin, Q. Hu, G. Ye, H. Shen, X. Wang, B. Z. Tang, W. A. Goddard, R. Ye, Nat. Synth., 2024, 3, 1231 56. K. Xu, J. Li, F. Liu, X. Chen, T. Zhao, F. Cheng, Angew. Chem. Int. Ed., 2023, 62, e202311968 57. Y. Pan, X. -Q. Li, G. -Y. Duan, J. Fang, B. -H. Xu, Appl. Catal. B, 2025, 361, 124681 58. H. -Q. Liang, S. Zhao, X. -M. Hu, M. Ceccato, T. Skrydstrup, K. Daasbjerg, ACS Catal., 2021, 11, 958 59. Z. Xing, L. Hu, D. S. Ripatti, X. Hu, X. Feng, Nat. Commun., 2021, 12, 136 60. X. Sheng, W. Ge, H. Jiang, C. Li, Adv. Mater., 2022, 34, 2201295 61. W. Nie, G. P. Heim, N. B. Watkins, T. Agapie, J. C. Peters, Angew. Chem. Int. Ed., 2023, 62, e202216102 62. Z. Liu, T. Yan, H. Shi, H. Pan, Y. Cheng, P. Kang, ACS Appl. Mater. Interfaces, 2022, 14, 7900 63. Z. Wang, W. Wang, Q. Yang, Y. Geng, Y. Du, K. Liu, Z. Wu, J. Lai, B. Li, H. Li, G. Zheng, L. Wang, Adv. Energy Mater., 2025, 1, 2405419 64. Q. Fan, G. Bao, X. Chen, Y. Meng, S. Zhang, X. Ma, ACS Catal., 2022, 1, 7517 65. T. Xu, H. Yang, T. Lu, R. Zhong, J. -J. Lv, S. Zhu, M. Zhang, Z. -J. Wang, Y. Yuan, J. Li, J. Wang, H. Jin, S. Pan, X. Wang, T. Cheng, S. Wang, Nat. Commun., 2025, 16, 977 66. H. Li, L. Fang, T. Wang, R. Bai, J. Zhang, T. Li, Z. Duan, K. -J. Chen, F. Pan, Adv. Mater., 2024, 1, 2416337 67. B. Sun, Z. Li, D. Xiao, H. Liu, K. Song, Z. Wang, Y. Liu, Z. Zheng, P. Wang, Y. Dai, B. Huang, A. Thomas, H. Cheng, Angew. Chem. Int. Ed., 2024, 63, e202318874 68. M. -D. Zhang, J. -R. Huang, W. Shi, P. -Q. Liao, X. -M. Chen, Angew. Chem. Int. Ed., 2023, 62, e202308195 69. H. Shen, H. Jin, H. Li, H. Wang, J. Duan, Y. Jiao, S. -Z. Qiao, Nat. Commun., 2023, 14, 2843 70. K. Yang, M. Li, T. Gao, G. Xu, D. Li, Y. Zheng, Q. Li, J. Duan, Nat. Commun., 2024, 15, 7060 71. Y. Wu, C. Chen, S. Liu, Q. Qian, Q. Zhu, R. Feng, L. Jing, X. Kang, X. Sun, B. Han, Angew. Chem. Int. Ed., 2024, 63, e202410659 72. M. Zhang, J. Wang, X. Rong, X. -L. Lu, T. -B. Lu, Nano Res., 2024, 17, 2381 73. W. Chen, R. Chen, Y. Jiang, Y. Wang, Y. Zhu, Y. Li, C. Li, Small, 2024, 20, 2306795 74. J. Feng, L. Wu, X. Song, L. Zhang, S. Jia, X. Ma, X. Tan, X. Kang, Q. Zhu, X. Sun, B. Han, Nat. Commun., 2024, 15, 4821 75. Y. Yang, J. Zhang, Z. Tan, J. Yang, S. Wang, M. Li, Z. Su, Angew. Chem. Int. Ed., 2024, 63, e202408873 76. T. Zhang, J. C. Bui, Z. Li, A. T. Bell, A. Z. Weber, J. Wu, Nat. Catal., 2022, 5, 202 77. T. Zhang, Z. Li, J. Zhang, J. Wu, J. Catal., 2020, 387, 163 78. X. She, T. Zhang, Z. Li, H. Li, H. Xu, J. Wu, Cell Rep. Phys. Sci., 2020, 1, 100051 79. F. -Z. Li, H. -G. Qin, H. -L. Zhang, X. Yue, L. -K. Fu, B. Xu, M. Lin, J. Gu, Joule, 2024, 8, 1772 80. M. Sun, J. Cheng, M. Yamauchi, Nat. Commun., 2024, 15, 491 81. K. Lv, C. Teng, M. Shi, Y. Yuan, Y. Zhu, J. Wang, Z. Kong, X. Lu, Y. Zhu, Adv. Funct. Mater., 2018, 28, 1802339 82. T. Shi, D. Liu, N. Liu, Y. Zhang, H. Feng, Q. Li, Adv. Sci., 2022, 9, 2204472 83. Y. Xu, J. P. Edwards, S. Liu, R. K. Miao, J. E. Huang, C. M. Gabardo, C. P. O’Brien, J. Li, E. H. Sargent, D. Sinton, ACS Energy Lett., 2021, 6, 809 84. X. Zhao, H. Xie, B. Deng, L. Wang, Y. Li, F. Dong, Chem. Commun., 2024, 60, 542 85. X. Yu, Y. Xu, L. Li, M. Zhang, W. Qin, F. Che, M. Zhong, Nat. Commun., 2024, 15, 1711 86. L. Fan, F. Li, T. Liu, J. E. Huang, R. K. Miao, Y. Yan, S. Feng, C. -W. Tai, S. -F. Hung, H. -J. Tsai, M. -C. Chen, Y. Bai, D. Kim, S. Park, P. Papangelakis, C. Wu, A. Shayesteh Zeraati, R. Dorakhan, L. Sun, D. Sinton, E. Sargent, Nat. Synth., 2025, 4, 262 87. Y. Xu, R. K. Miao, J. P. Edwards, S. Liu, C. P. O’Brien, C. M. Gabardo, M. Fan, J. E. Huang, A. Robb, E. H. Sargent, D. Sinton, Joule, 2022, 6, 1333 88. Z. Mi, T. Wang, L. Xiao, G. Wang, L. Zhuang, J. Am. Chem. Soc., 2024, 146, 17377 89. D. M. Koshy, S. A. Akhade, A. Shugar, K. Abiose, J. W. Shi, S. W. Liang, J. S. Oakdale, S. E. Weitzner, J. B. Varley, E. B. Duoss, S. E. Baker, C. Hahn, Z. N. Bao, T. F. Jaramillo, J. Am. Chem. Soc., 2021, 143, 14712 90. E. Vichou, A. Perazio, Y. Adjez, M. Gomez-Mingot, M. W. Schreiber, C. M. Sánchez-Sánchez, M. Fontecave, Chem. Mater., 2023, 35, 7060 91. C. Kim, J. C. Bui, X. Y. Luo, J. K. Cooper, A. Kusoglu, A. Z. Weber, A. T. Bell, Nat. Energy, 2021, 6, 1026 92. Z. Gao, L. Xue, X. Hu, J. Yin, L. Xiao, G. Wang, J. Lu, L. Zhuang, Electrochim. Acta, 2023, 458, 142509 93. S. Zhong, X. Yang, Z. Cao, X. Dong, S. M. Kozlov, L. Falivene, J. -K. Huang, X. Zhou, M. N. Hedhili, Z. Lai, K. -W. Huang, Y. Han, L. Cavallo, L. -J. Li, Chem. Commun., 2018, 54, 11324 94. B. Wu, B. Wang, B. Cai, C. Wu, W. W. Tjiu, M. Zhang, Z. Aabdin, S. Xi, Y. Lum, J. Am. Chem. Soc., 2024, 146, 29801 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Advantages and challenges for acidic CO2RR. Schematic illustrations of a carbonate generation under alkaline condition. b carbonate regeneration under acidic condition. c challenges of competitive hydrogen evolution reaction under acidic condition, and d carbon-carbon coupling under acidic condition.
-
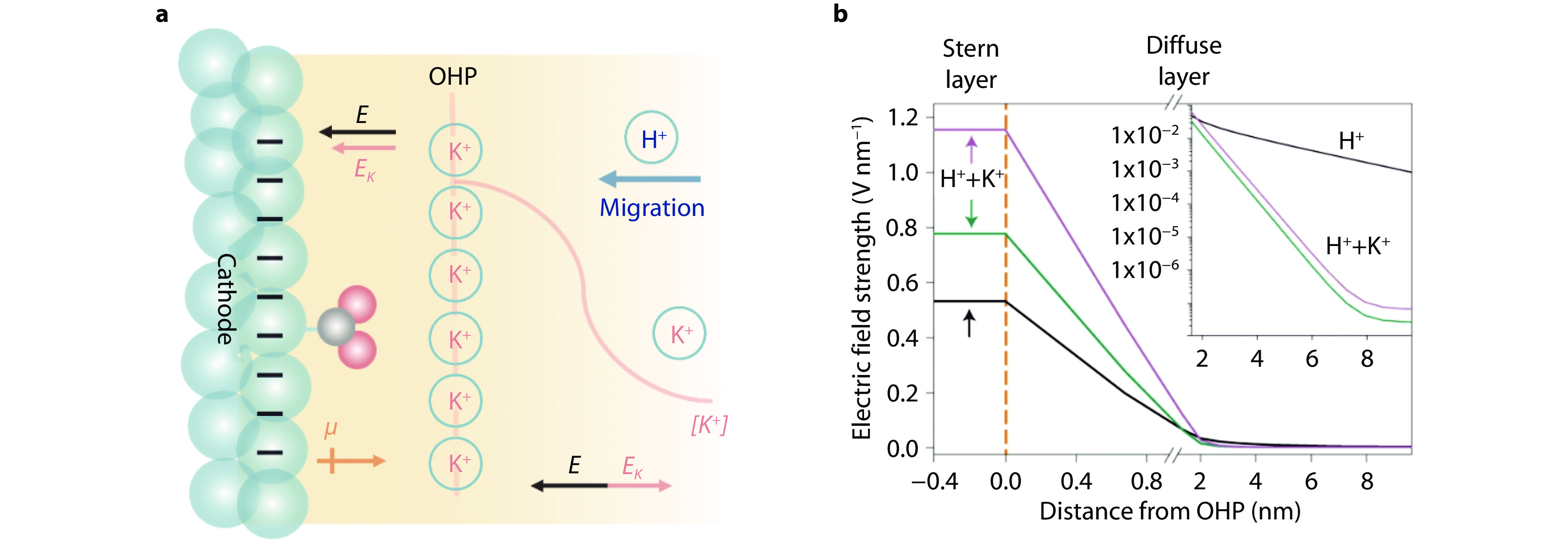
Figure 2.
Roles of K+ for CO2RR under acidic conditions. a Schematic illustration of K+ under acidic condition. E represents the electric field generated by cathode potential; EK represents the electric field generated by K+ at outer Helmholtz plane; μ represents the dipole moment of the adsorbed CO2 intermediate; OHP is outer Helmholtz plane. b Strength of electric field profiles over the distance from OHP. Black, green and pink curves represent profiles obtained in 0.1 M HOTf at − 0.7 V, 0.1 M HOTf + 0.4 M KOTf at − 0.7 V, and 0.1 M HOTf + 0.4 M KOTf at −1.1 V (vs. potential of zero charge (PZC)), respectively. [21] Copyright 2022, Springer Nature.
-
Figure 3.
Augmenting local K+ concentration for acidic CO2RR. a LSV curves on Au rotating disk electrode (RDE) in Ar-saturated 10 mM HClO4 with 0-100 mM LiClO4. The electric field strength in the Stern layer as a function of electrode potential b in 10 mM HClO4 with 0-100 mM LiClO4, and c in 10 mM HClO4 with 10 mM MClO4 (M = Li, Na, K, Cs). [42] Copyright 2023, American Chemical Society. d TEM image of Cu nanoneedles (inset) and electric field distribution near Cu nanoneedles surface simulated by COMSOL; e FEs of CO2RR products on Cu nanoneedles at 0.8 to 1.6 A cm−2 under acidic condition (3 M KCl, pH = 1). [53] Copyright 2023, Wiley-VCH. f K+ distribution on electrochemically reduced Cu porous nanosheets simulated by COMSOL. g FEs and current densities of CO2RR products on porous Cu nanosheets under acidic condition (0.05 H2SO4 with 3 M KCl). [47] Copyright 2022, Springer Nature. h Schematic illustration of local environment and ions transport near the PFSA modified Cu surface (denoted as Cu/PFSA); i FEs of CO2RR products at 0.4 to 1.5 A cm−2 on Cu/PFSA.[18] Copyright 2021, American Association for the Advancement of Science.
-
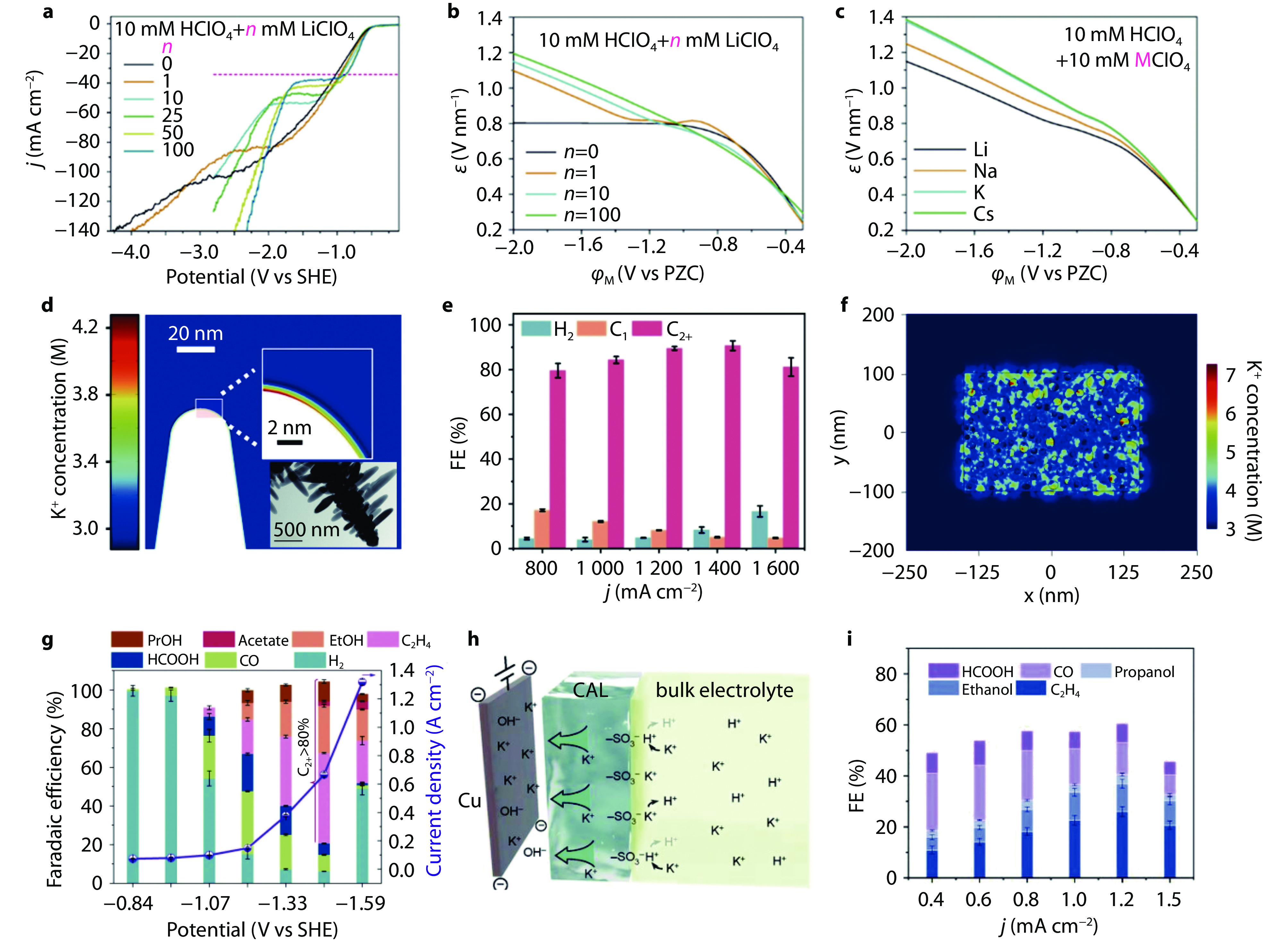
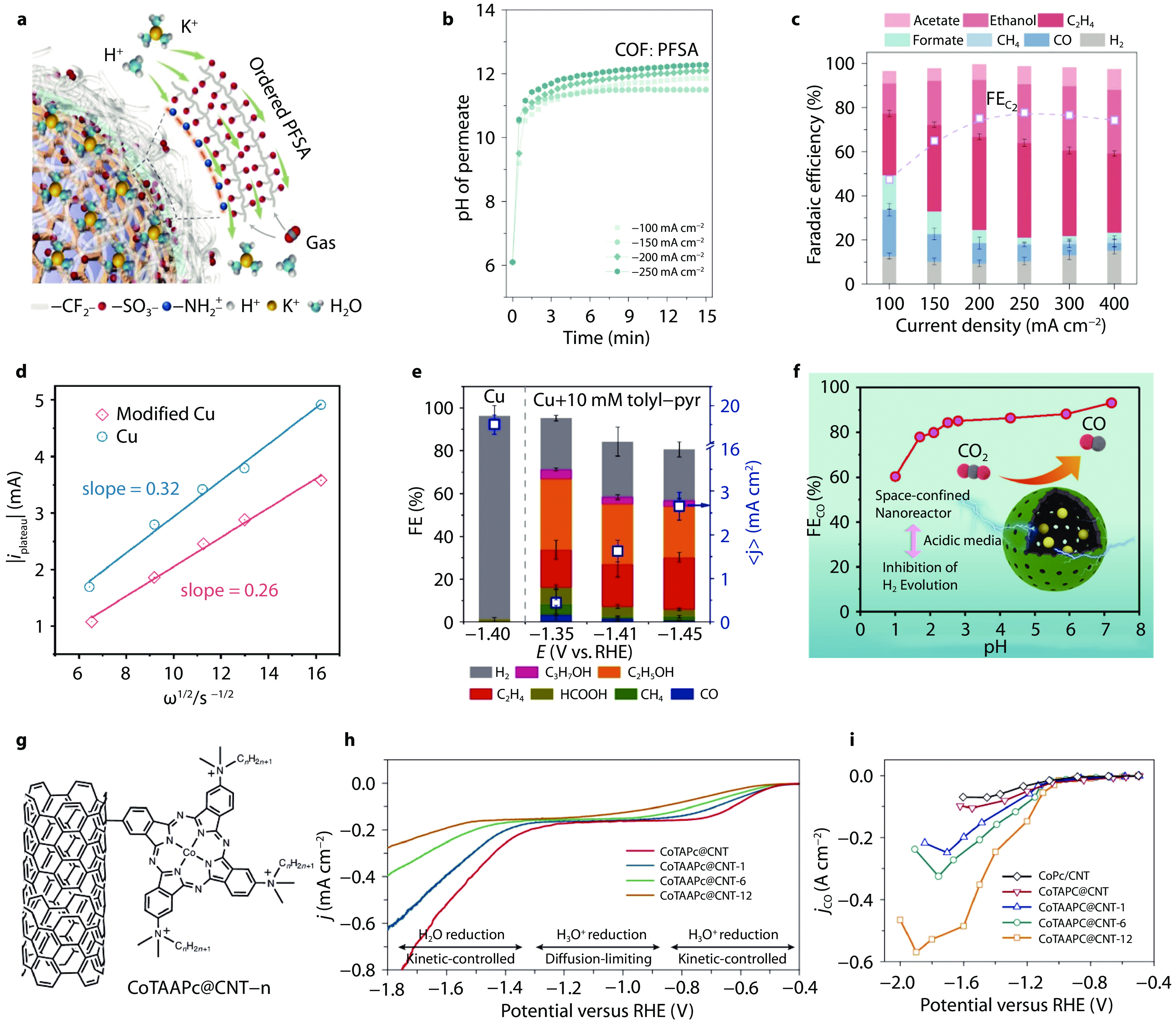
Figure 4.
Restricting H+/H2O mass transport for acidic CO2RR. a Schematic illustration of COF:PFSA layer on catalyst surface and local ions/gas transport; b pH change of permeate during 15 min of electrolysis at 100-250 mA cm−2 for COF:PFSA-modified electrodes; c FEs of various CO2RR products at 100-400 mA cm−2 on COF:PFSA-modified Cu electrode under acidic condition (pH = 1).[4] Copyright 2023, Springer Nature. d Limiting diffusion current of H+ reduction on Cu and organic film modified Cu RDEs in acidic HClO4-KClO4 electrolyte (pH = 2.2); the slop represents the diffusion coefficient of H+; e FEs and current densities of various CO2RR products on Cu electrode in acidic electrolyte (pH = 2) with and without tolyl-pyr additive. [61] Copyright 2023, Wiley-VCH. f Schematic illustration of Ni nanoparticles encapsulated in N-doped carbon nanocages and FEs of CO as a function of electrolyte pH at −1.4 V vs Ag/AgCl.[62] Copyright 2022, American Chemical Society. g Scheme of CoTAAPc@CNT-n (where n denotes the length of alkyl chains in the ammonium groups, n = 1, 6 and 12). h LSV curves of CoTAAPc@CNT-12-n in N2-saturated electrolyte of 0.05 M H2SO4 and 0.5 M K2SO4 at 1,600 rpm. i Carbon monoxide partial current density curves of CoTAAPc@CNT-n in 0.05 M H2SO4 + 3.0 M KCl. [55] Copyright 2024, Springer Nature.
-
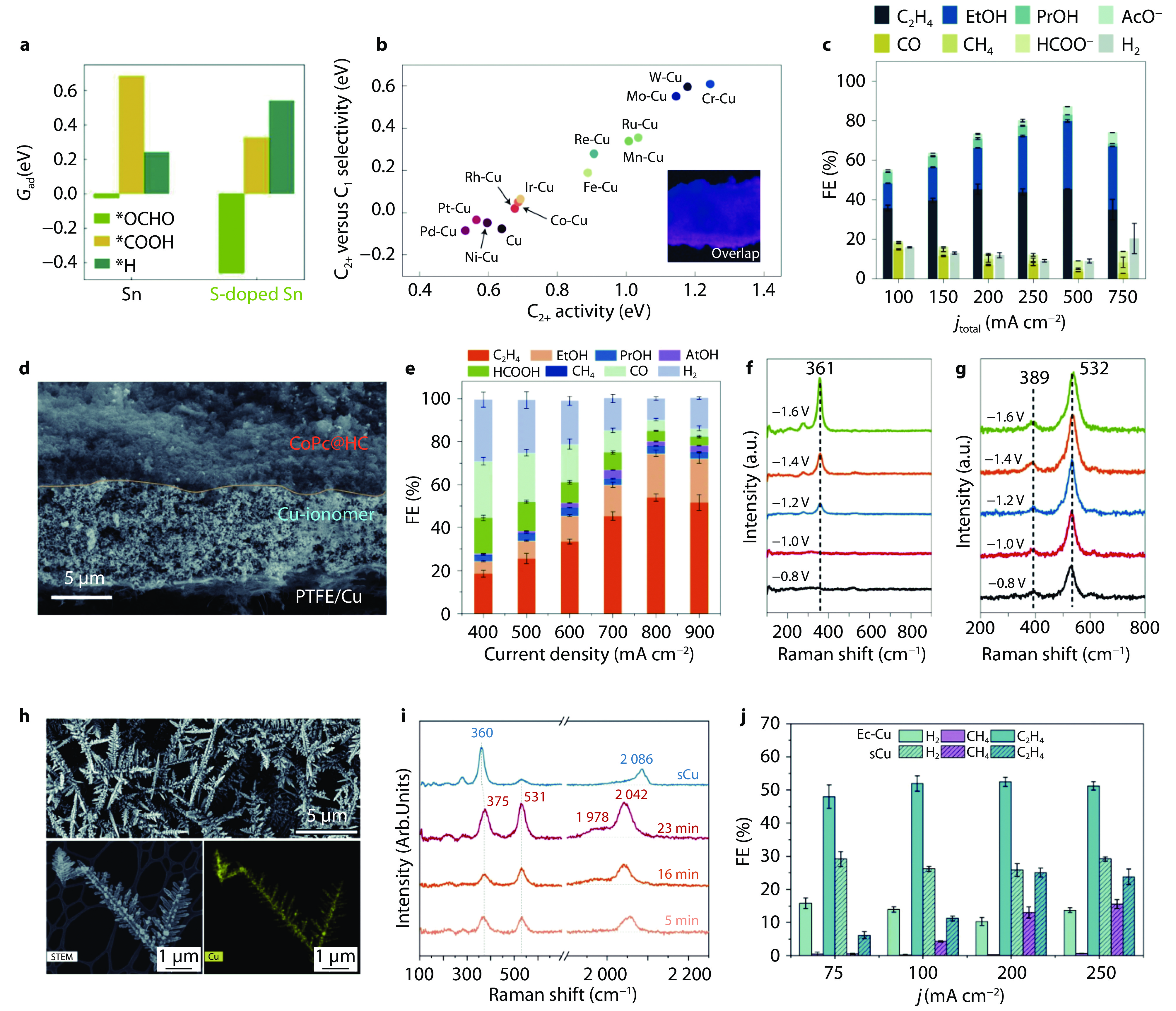
Figure 5.
Enhancing the intrinsic CO2RR activity of catalysts in acid. a Adsorption energy of *H, *COOH, and *OCHO intermediates on Sn surface and S-doped Sn surface. [69] Copyright 2023, Springer Nature. b Two-dimensional C2+ activity (∆GOCCOH*) and C2+ versus C1 selectivity (∆GOCCOH*-∆GCHO*) profile of CO2 electrolysis to C1 and C2+ products (inset shows the overlapped elemental mapping of Pd-Cu catalyst). c FEs of various CO2RR products on Pd-Cu electrode at 0.1-0.75 A cm−2 in 0.5 M K2SO4 electrolyte (pH = 2).[26] Copyright 2022, Springer Nature. d Cross-section scanning electron microscopy (SEM) image of CoPc@HC/Cu tandem electrode. e FEs of various CO2RR products at 0.4-0.9 A cm−2 on CoPc@HC/Cu tandem electrode in an acidic electrolyte containing 2.5 M KCl, 0.5 M KH2PO4 and 0.5 M H3PO4 (pH = 1). f-g In situ Raman spectra of f Cu electrode and g CoPc/Cu tandem electrode, respectively, at various cathode potentials.[14] Copyright 2023, Springer Nature. h SEM and scanning transmission electron microscopy (STEM) images of EC-Cu and corresponding EDX mapping. i In situ Raman spectra of sCu and EC–Cu with different electrodeposition times. j FEs toward electrolysis gaseous products on EC-Cu and sCu at 75-250 mA cm−2 under acidic condition.[27] Copyright 2023, Springer Nature.
-
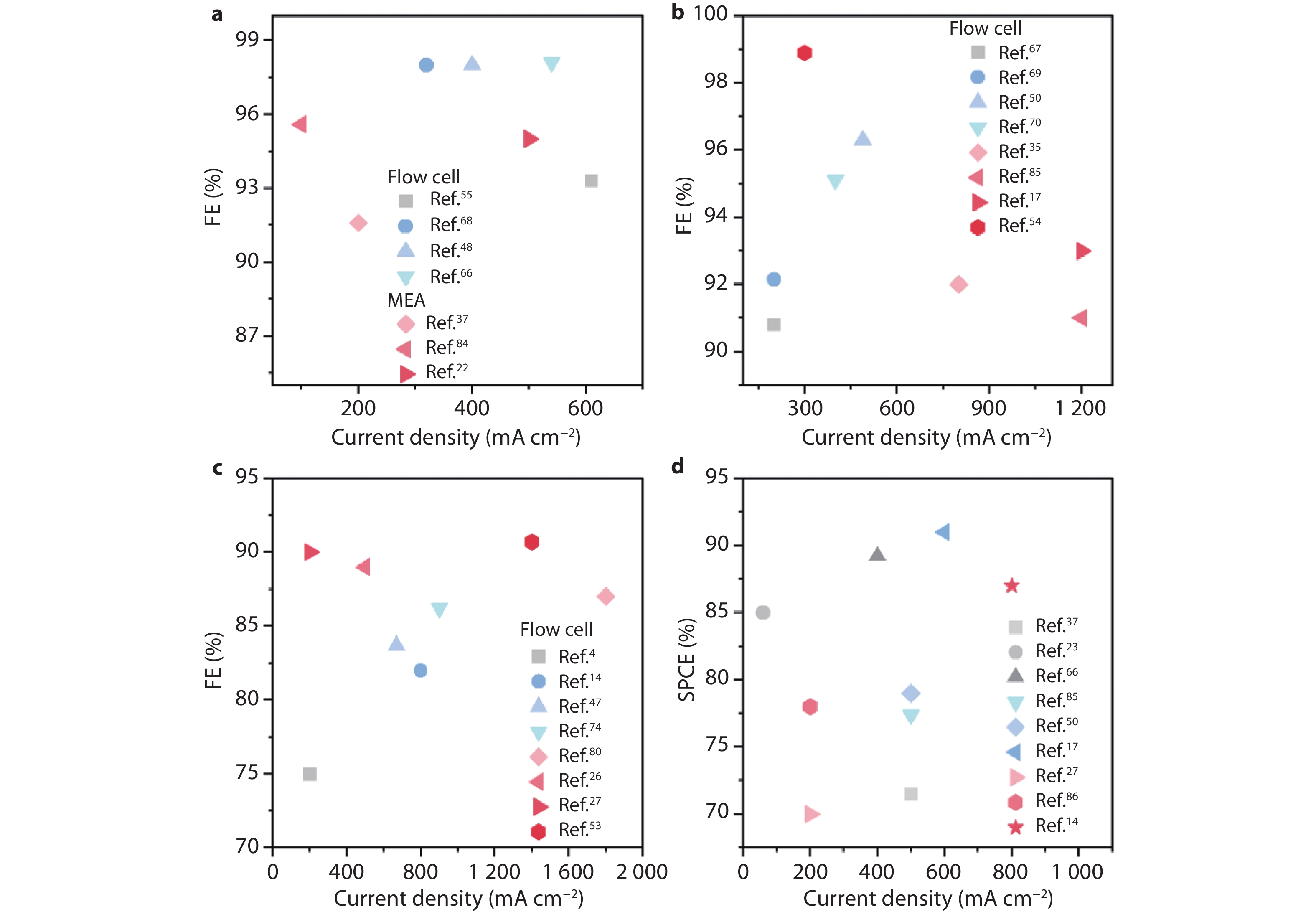
Figure 6.
Summary of CO2RR performance under K+ participating acidic conditions. a FEs vs. total current densities for a CO,[22,37,48,55,66,68,84] b HCOOH,[17,35,50,54,67,69,70,85] c C2+ products.[4,14,26,27,47,53,74,80] d SPCEs vs. total current densities for different products (Black: CO; blue: HCOOH; red: C2+ products).[14,17,23,27,37,50,66,85,86]
-
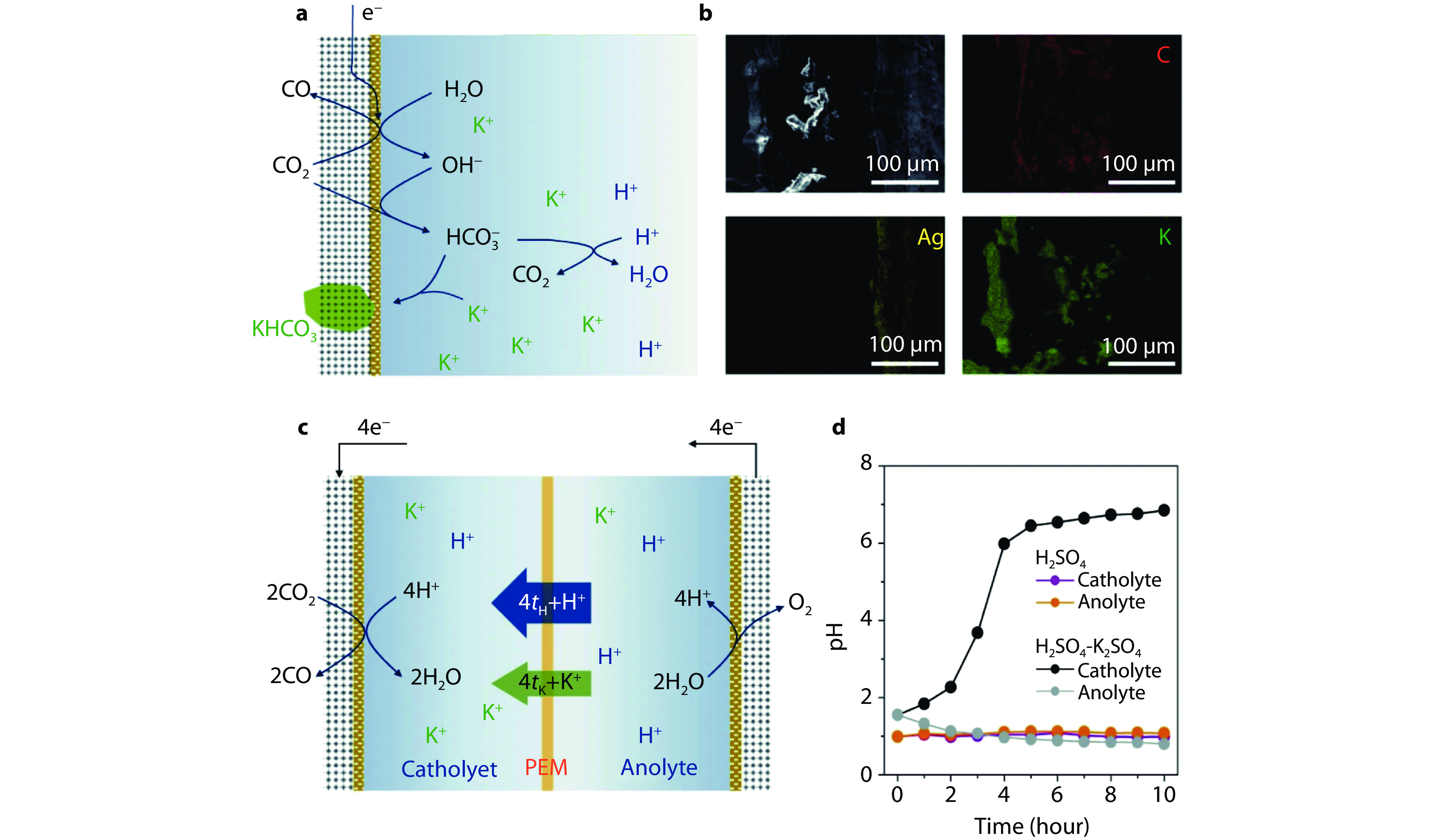
Figure 7.
Challenges of acidic CO2 electroreduction in alkali-metal-cation-containing electrolytes. a Schematic illustration of the (bi)carbonate precipitation on GDE in acidic electrolytes containing K+. b Cross-section SEM image and corresponding EDS mappings of Ag-GDE electrode after CO2RR test in 0.1 M H2SO4 with 0.4 M K2SO4. c Schematic illustration of the cations migration from anode to cathode in acidic electrolytes containing K+. d The pH values of catholyte and anolyte as a function of electrolysis duration at 200 mA cm−2 in acidic electrolytes with and without K+. [24] Copyright 2023, Springer Nature.
-
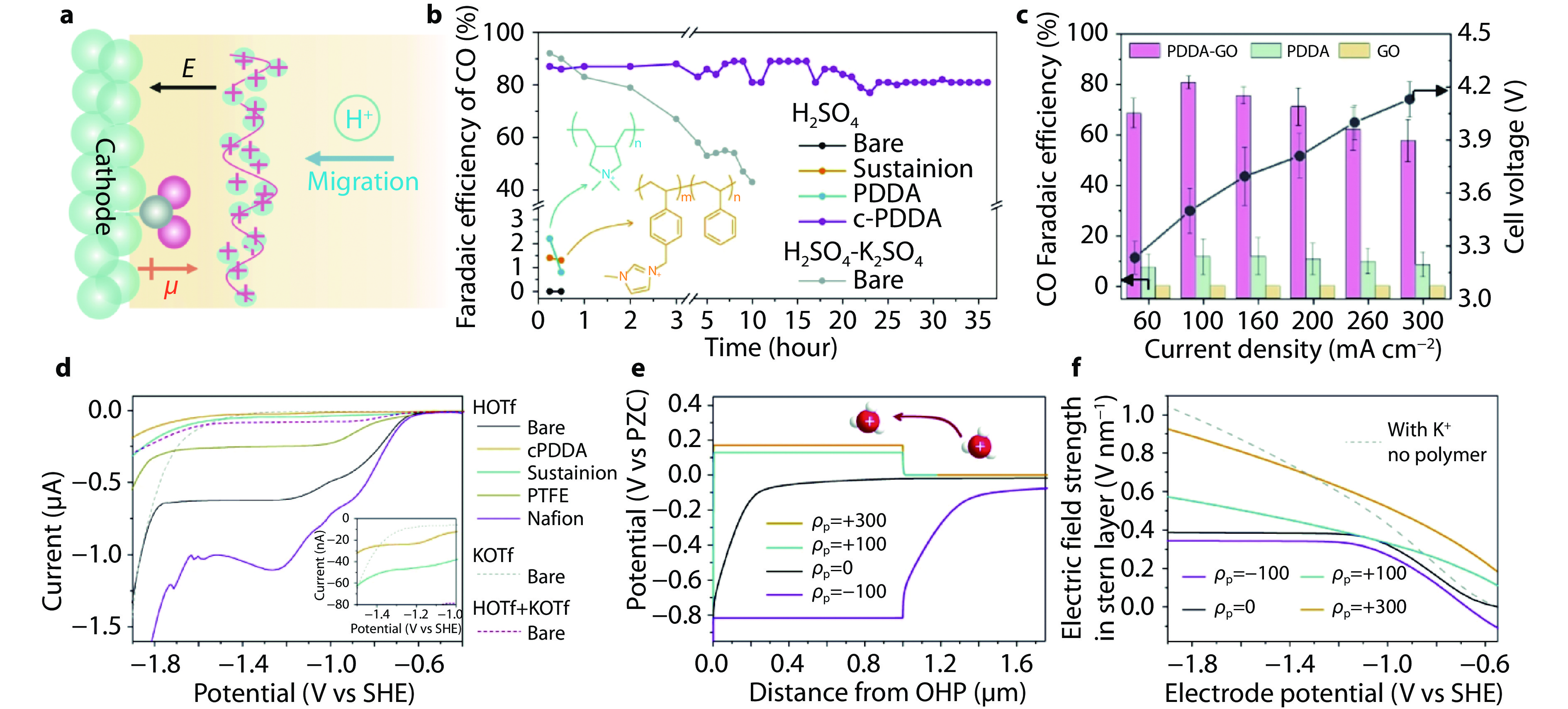
Figure 8.
PDDA-based organic cations for acidic CO2RR. a Schematic illustration of immobilized cationic modification. b FEs of CO on bare Ag in 0.1 M H2SO4 + 0.4 M K2SO4 electrolyte and cationic-polyelectrolyte-modified Ag electrodes in 0.1 M H2SO4 electrolyte. Copyright 2023, Springer Nature. c FEs of CO and cell voltages on PDDA-GO, PDDA, and GO-modified Ag electrodes in acidic MEA with 0.01 M H2SO4. [39] Copyright 2024, Wiley-VCH. d HER polarization curves of Ag MDEs in 10mM HOTf (solid curves): bare Ag MDE (black), Ag MDEs modified by c-PDDA (orange), Sustainion XA-9 (blue), PTFE (dark yellow) and Nafion D520 (magenta). The gray and red dashed curves show the HER polarization curves of bare Ag MDE in 10 mM KOTf and 10 mM HOTf + 10 mM KOTf, respectively. e Potential profiles on Ag electrodes covered by polymer layers with different ρp in 10 mM HOTf. The electrode potential is −1.8 V vs SHE and ρp is the charge density carried by the polymer layer. f Plots of the electric field strength in the Stern layer based on the electrode potential. Solid curves: Ag electrodes covered by polymer layers with different ρp in 10 mM HOTf. Gray dashed curve: bare Ag electrode in 10 mM HOTf + 40 mM KOTf. [24] Copyright 2023, Springer Nature.
-
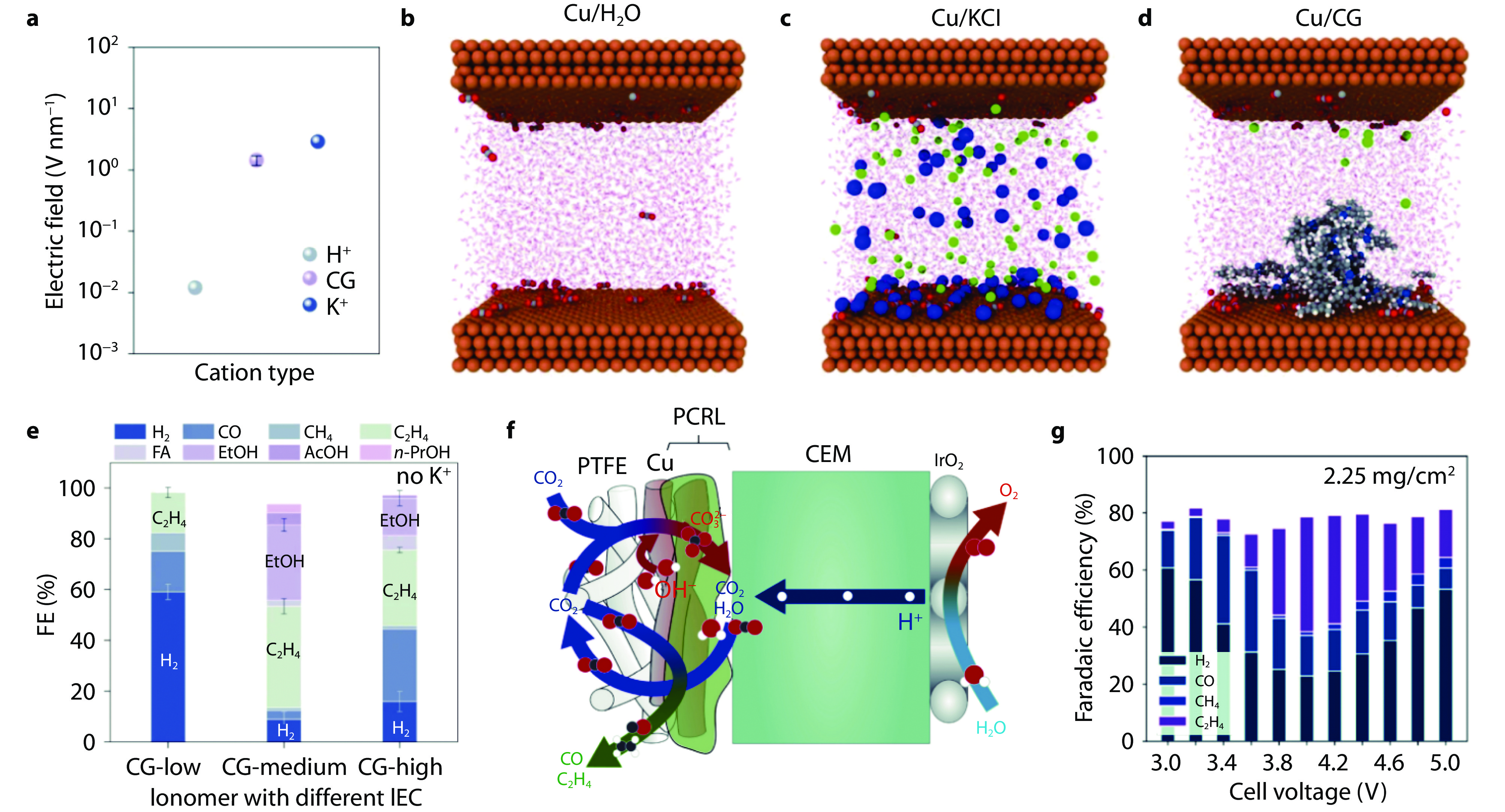
Figure 9.
Imidazolium-based and quaternary ammonium organic cations for acidic CO2RR. a Distribution of electric field with different cations at OHP. Molecular dynamics model predictions of atomic configurations for Cu/H2O b, Cu/KCl c, and Cu/CG d systems at the end of NVE simulation (ΔU = 2.5 V). Red, grey, yellow, green, purple, blue, and white balls represent O, C, Cu, Cl, K, N, and H, respectively. e FFs of various CO2RR products on various CG- modified Cu electrodes at 100 mA cm−2 in 0.2 M H2SO4. [16] Copyright 2024, Springer Nature. f Schematic illustration of the mass transport and reaction on anion exchange ionomers with positive charged quaternary ammonium CG (denoted as PCRL) modified Cu/PTFE electrode in CEM-based MEA. g FEs of gaseous products on PCRL-modified Cu/PTFE electrode at different cell voltages.[11] Copyright 2021, American Chemical Society.
-
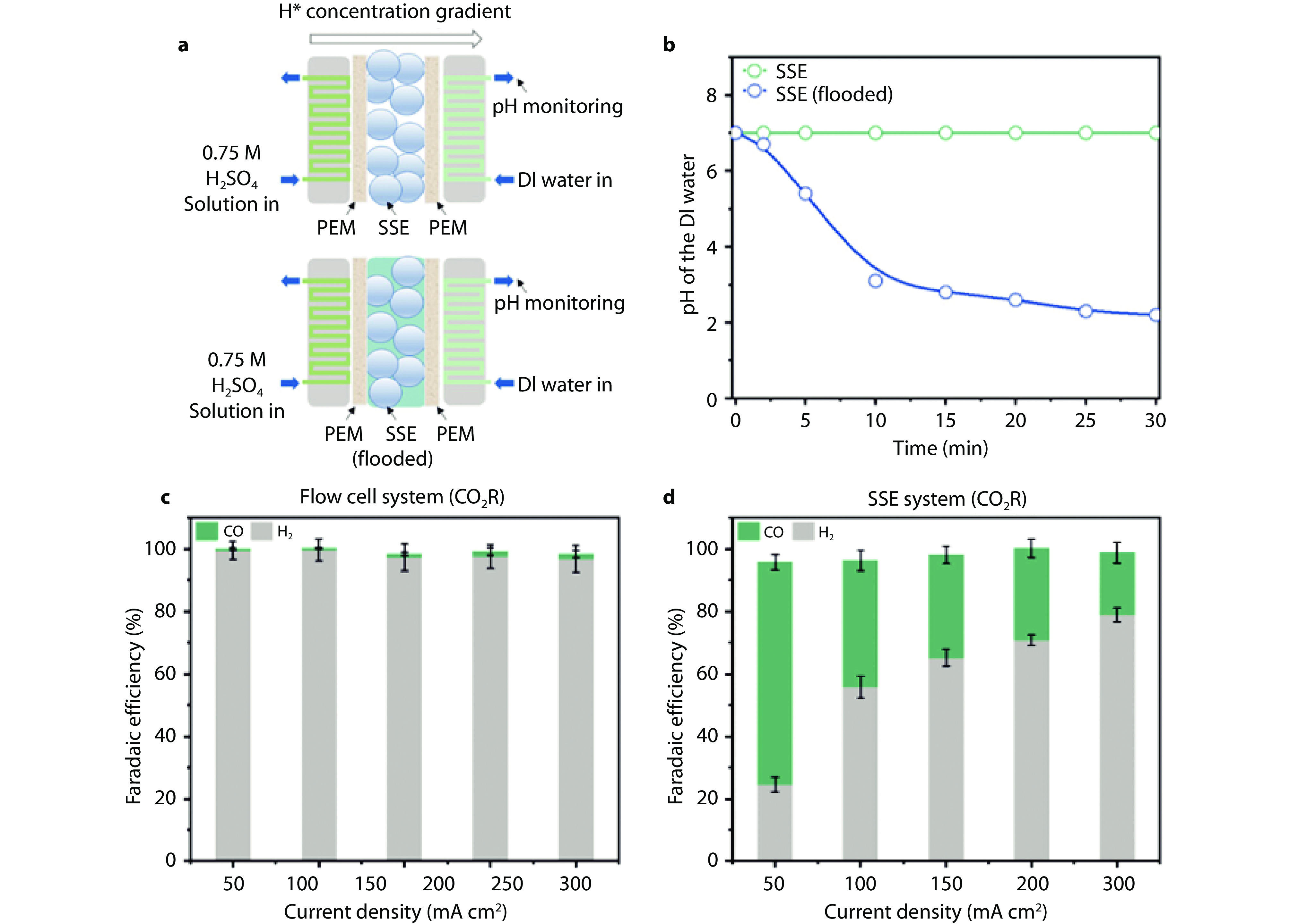
Figure 10.
Reducing surface H+ concentration in pure acidic conditions. a Schematic of the experimental device to examine H+ diffusion through the solid-state electrolyte (SSE). The top one is operating with only SSE and the bottom one is operating with flooded SSE (water + SSE). b Bulk pH of deionized water changes versus time for both the SSE and the flooded SSE. c FEs on Au/PTFE under different current densities in 0.25 M H2SO4 in flow cell. d FEs on Au/PTFE under different current densities in MEA with SSE with 0.25 M H2SO4 used as the electrolyte.[94] Copyright 2024, American Chemical Society.
-
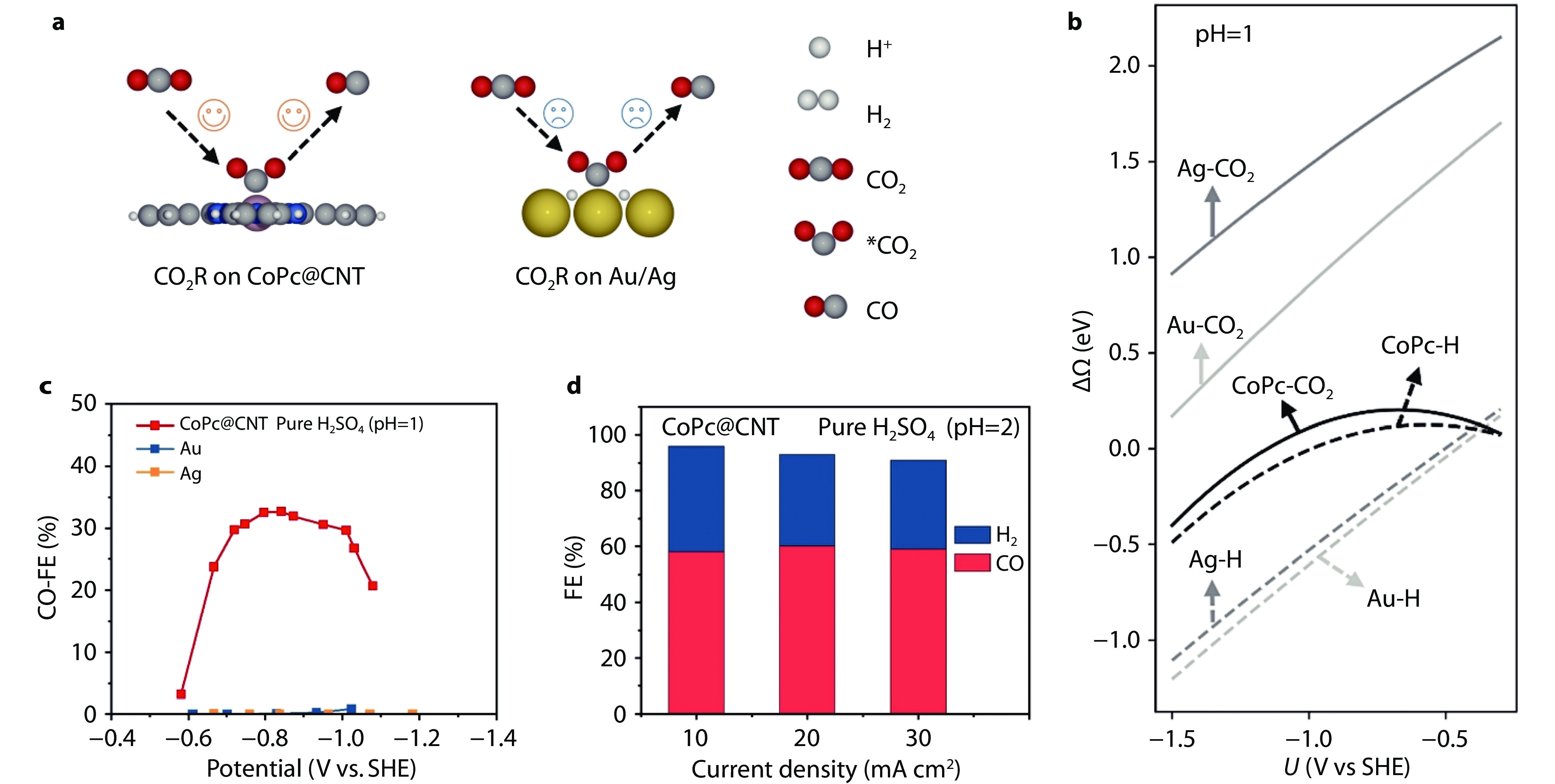
Figure 11.
Electrocatalyst design for CO2RR in pure acids. a Schematic illustration of *CO2 and *H absorptions on CoPc@CNT and Au/Ag surface. b Adsorption free energies of *H and *CO2 on Au, Ag, and CoPc@CNT as a function of potential at pH = 1. c FEs of CO on CoPc@CNT, Ag, and Au under different potentials in pure H2SO4 (pH = 1). d FEs of CO on CoPc@CNT under different current densities in pure H2SO4 (pH = 2).[36] Copyright 2024, Wiley-VCH.

 Mr. Yue Yang received his bachelor degree in material engineering from Northeastern University, China in 2021. He is currently a PhD candidate in Prof. Hao Bin Wu’s group at Zhejiang University, China. His research focuses on electrochemical carbon dioxide reduction.
Mr. Yue Yang received his bachelor degree in material engineering from Northeastern University, China in 2021. He is currently a PhD candidate in Prof. Hao Bin Wu’s group at Zhejiang University, China. His research focuses on electrochemical carbon dioxide reduction.  Dr. Xiaotong Li received her Ph.D. degree in Materials Science and Engineering from Zhejiang University in 2023 under the supervision of Professor Hao Bin Wu. She is currently a postdoctoral fellow at Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Her research focuses on the rational design and synthesis of copper-based electrocatalysts for efficient and selective electrochemical CO2 reduction.
Dr. Xiaotong Li received her Ph.D. degree in Materials Science and Engineering from Zhejiang University in 2023 under the supervision of Professor Hao Bin Wu. She is currently a postdoctoral fellow at Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Her research focuses on the rational design and synthesis of copper-based electrocatalysts for efficient and selective electrochemical CO2 reduction.  Dr. Hao Bin Wu is a Tenured Associate Professor at the School of Materials Science and Engineering, Zhejiang University. He received his B.S. degree from Fudan University in 2010, and Ph.D. from Nanyang Technological University in 2015. He conducted postdoctoral research at the University of California, Los Angeles, before joining Zhejiang University in 2017. Dr. Wu’s research focuses on advanced electrochemical energy storage and conversion technologies, with particular emphasis on lithium batteries and CO2 electrolysis. He has co-authored over 170 publications with an H-index of 93. He was recognized as a Clarivate Analytics’ Highly Cited Researcher from 2017 to 2024.
Dr. Hao Bin Wu is a Tenured Associate Professor at the School of Materials Science and Engineering, Zhejiang University. He received his B.S. degree from Fudan University in 2010, and Ph.D. from Nanyang Technological University in 2015. He conducted postdoctoral research at the University of California, Los Angeles, before joining Zhejiang University in 2017. Dr. Wu’s research focuses on advanced electrochemical energy storage and conversion technologies, with particular emphasis on lithium batteries and CO2 electrolysis. He has co-authored over 170 publications with an H-index of 93. He was recognized as a Clarivate Analytics’ Highly Cited Researcher from 2017 to 2024. 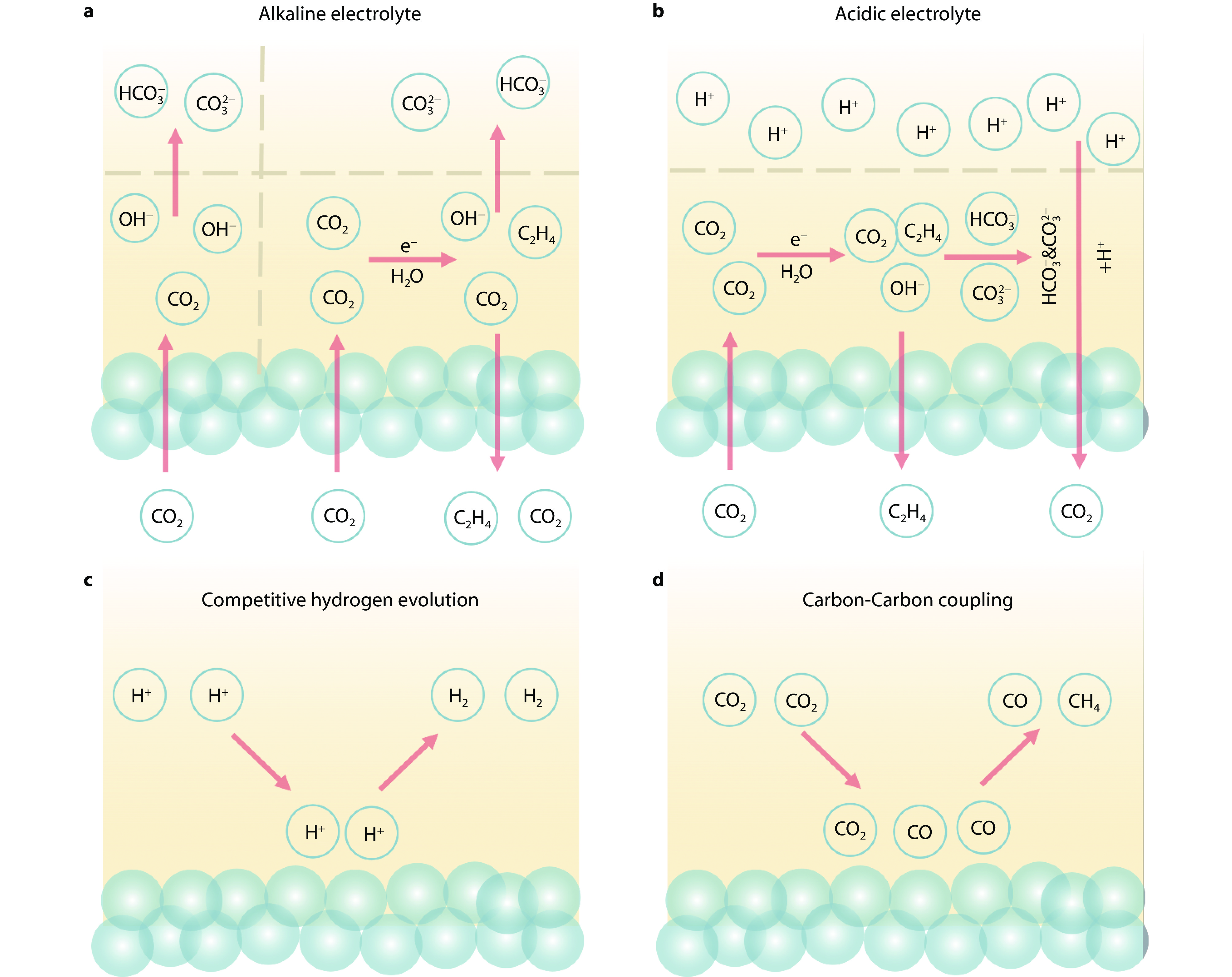

 DownLoad:
DownLoad: