| Citation: | Songliang Liu, Shuli Yin, Lin Cui, Hongjie Yu, Kai Deng, Ziqiang Wang, You Xu, Liang Wang, Hongjing Wang. Interface engineering-inspired electron regulation in Pt/Pd hetero-metallene for methanol-assisted hydrogen evolution[J]. Energy Lab, 2023, 1(1): 220005. doi: 10.54227/elab.20220005 |
Interface engineering-inspired electron regulation in Pt/Pd hetero-metallene for methanol-assisted hydrogen evolution
-
Abstract
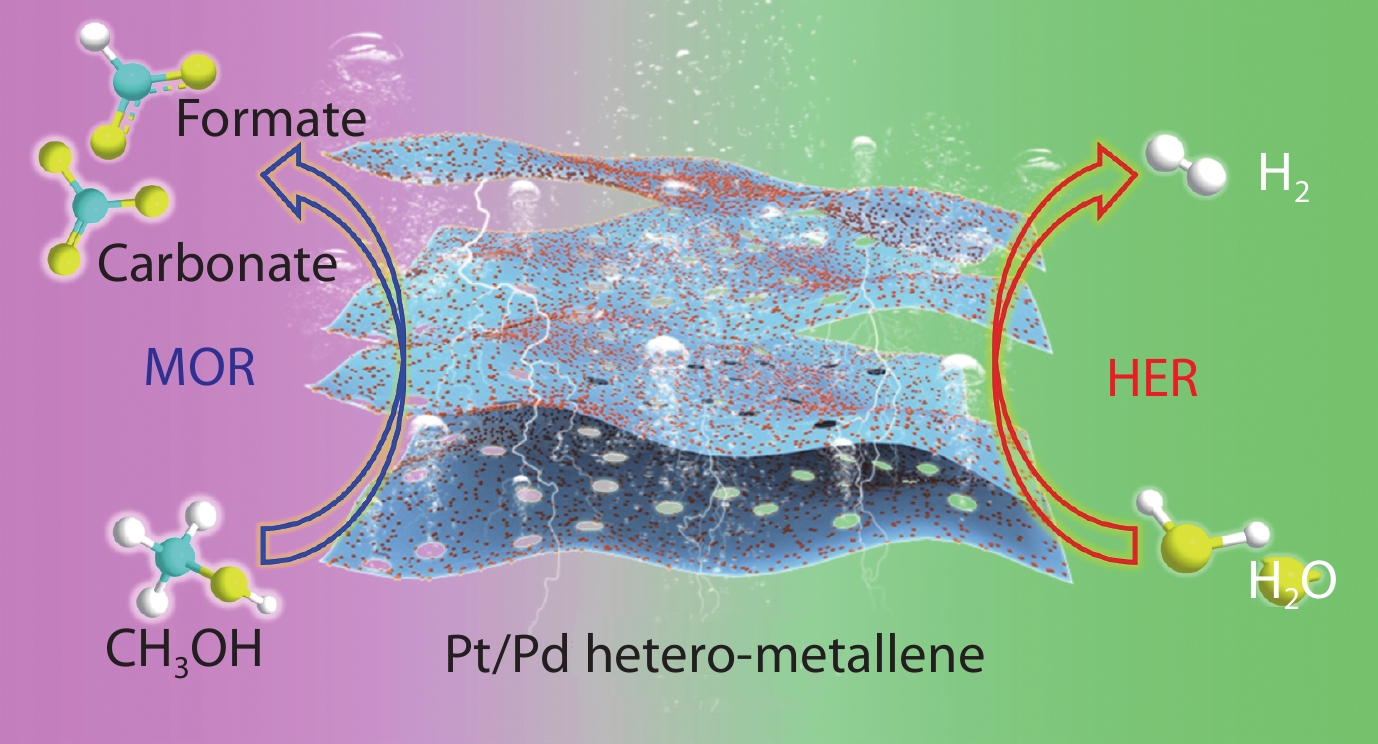
The small molecule oxidation reaction instead of oxygen evolution reaction coupled with hydrogen evolution reaction can greatly reduce the reaction overpotential of electrochemical water splitting, which is a very efficient and energy-saving hydrogen evolution strategy. Herein, we report an interface engineering constructed two-dimensional ultrathin curled Pt/Pd hetero-metallene for efficient electrocatalytic hydrogen evolution assisted by methanol. The thin-sheet structure of Pt/Pd hetero-metallene provides a large specific surface area and exposes numerous surface atoms that could act as reactive sites, thus accelerating the reaction mass transfer process. More importantly, the constructed Pt/Pd hetero-metallene possesses abundant Pt/Pd heterointerface, which can maximize the strong metal-metal interaction and increase the utilization of metal atoms, thereby optimizing the adsorption and activation of reactants during the reaction. Pt/Pd hetero-metallene can produce hydrogen stably and efficiently in 1 M KOH + 1 M CH3OH, and the voltage only needs 0.83 V at @100 mA cm−2 when used in electrocatalytic hydrogen evolution, which is much lower than the voltage required for the traditional electrochemical water splitting process (1.94 V). This work not only provides a powerful approach to rational design and construction of hetero-metallene through interface engineering, but also builds a bridge between hetero-metallene and methanol-assisted hydrogen evolution.
-
-
References
1. Z. Zhao, H. Liu, W. Gao, W. Xue, Z. Liu, J. Huang, X. Pan, Y. Huang, J. Am. Chem. Soc., 2018, 140, 9046 2. Y. Li, C. K. Peng, H. Hu, S. Y. Chen, J. H. Choi, Y. G. Lin, J. Lee, M, Nat. Commun., 2022, 13, 1143 3. Z. L. Wang, K. Sun, J. Henzie, X. Hao, C. Li, T. Takei, Y. M. Kang, Y. Yamauchi, Angew. Chem. Int. Ed., 2018, 57, 5848 4. K. Wang, B. Huang, F. Lin, F. Lv, M. Luo, P. Zhou, Q. Liu, W. Zhang, C. Yang, Y. Tang, Y. Yang, W. Wang, H. Wang, S. Guo, Adv. Energy Mater., 2018, 8, 1801891 5. Y. Guo, J. Tang, Z. Wang, Y.-M. Kang, Y. Bando, Y. Yamauchi, Nano Energy, 2018, 47, 494 6. X. Wu, J. Li, Y. Li, Z. Wen, Chem. Eng. J., 2021, 409, 128161 7. M. Zhang, J. Chen, H. Li, P. Cai, Y. Li, Z. Wen, Nano Energy, 2019, 61, 576 8. Y. Xu, X. Chai, T. Ren, S. Yu, H. Yu, Z. Wang, X. Li, L. Wang, H. Wang, Chem. Commun., 2020, 56, 2151 9. S. Fang, X. Zhu, X. Liu, J. Gu, W. Liu, D. Wang, W. Zhang, Y. Lin, J. Lu, S. Wei, Y. Li, T. Yao, Nat. Commun., 2020, 11, 1029 10. A. Ali, P. K. Shen, Carbon Energy, 2019, 2, 99 11. Q. Mao, K. Deng, W. Wang, P. Wang, Y. Xu, Z. Wang, X. Li, L. Wang, H. Wang, J. Mater. Chem. A, 2022, 10, 8364 12. W. Zhang, B. Huang, K. Wang, W. Yang, F. Lv, N. Li, Y. Chao, P. Zhou, Y. Yang, Y. Li, J. Zhou, W. Zhang, Y. Du, D. Su, S. Guo, Adv. Energy Mater., 2020, 11, 2003192 13. J. Xu, I. Amorim, Y. Li, J. Li, Z. Yu, B. Zhang, A. Araujo, N. Zhang, L. Liu, Carbon Energy, 2020, 2, 646 14. H. Huang, Y. Zhao, Y. Bai, F. Li, Y. Zhang, Y. Chen, Adv. Sci., 2020, 7, 2000012 15. G. Zhou, M. Li, Y. Li, H. Dong, D. Sun, X. Liu, L. Xu, Z. Tian, Y. Tang, Adv. Funct. Mater., 2019, 30, 1905252 16. W. Song, M. Li, C. Wang, X. Lu, Carbon Energy, 2020, 3, 101 17. K. Kani, H. Lim, A. E. Whitten, K. Wood, A. J. E. Yago, M. S. A. Hossain, J. Henzie, J. Na, Y. Yamauchi, J. Mater. Chem. A, 2021, 9, 2754 18. W. Zhou, T. Jia, D. Zhang, Z. Zheng, W. Hong, X. Chen, Appl. Catal. B: Environ., 2019, 259, 118067 19. L. Chen, J. Shi, J. Mater. Chem. A, 2018, 6, 13538 20. K. Deng, Q. Mao, W. Wang, P. Wang, Z. Wang, Y. Xu, X. Li, H. Wang, L. Wang, Appl. Catal. B: Environ., 2022, 310, 121338 21. Y. Xu, M. Liu, S. Wang, K. Ren, M. Wang, Z. Wang, X. Li, L. Wang, H. Wang, Appl. Catal. B: Environ., 2021, 298, 120493 22. J. Y. Zhang, H. Wang, Y. Tian, Y. Yan, Q. Xue, T. He, H. Liu, C. Wang, Y. Chen, B. Y. Xia, Angew. Chem. Int. Ed., 2018, 57, 7649 23. C. Lin, P. Zhang, S. Wang, Q. Zhou, B. Na, H. Li, J. Tian, Y. Zhang, C. Deng, L. Meng, J. Wu, C. Liu, J. Hu, L. Zhang, Journal of Alloys and Compounds, 2020, 823, 153784 24. K. Xiang, D. Wu, X. Deng, M. Li, S. Chen, P. Hao, X. Guo, J. L. Luo, X. Z. Fu, Adv. Funct. Mater., 2020, 30, 1909610 25. G. Ma, X. Zhang, G. Zhou, X. Wang, Chem. Eng. J., 2021, 411, 128292 26. S. Yin, S. Liu, Z. Wang, Y. Xu, X. Li, H. Wang, L. Wang, Chem. Eng. J., 2022, 435, 134711 27. Y. Shi, Z. R. Ma, Y. Y. Xiao, Y. C. Yin, W. M. Huang, Z. C. Huang, Y. Z. Zheng, F. Y. Mu, R. Huang, G. Y. Shi, Y. Y. Sun, X. H. Xia, W. Chen, Nat. Commun., 2021, 12, 3021 28. J. Chen, M. Qin, S. Ma, R. Fan, X. Zheng, S. Mao, C. Chen, Y. Wang, Appl. Catal. B: Environ., 2021, 299, 120640 29. H.-S. Chen, T. M. Benedetti, J. Lian, S. Cheong, P. B. O’Mara, K. O. Sulaiman, C. H. W. Kelly, R. W. J. Scott, J. J. Gooding, R. D. Tilley, ACS Catal., 2021, 11, 2235 30. Z. Zhang, J. Liu, J. Wang, Q. Wang, Y. Wang, K. Wang, Z. Wang, M. Gu, Z. Tang, J. Lim, T. Zhao, F. Ciucci, Nat. Commun., 2021, 12, 5235 31. Q. Yang, H. Liu, P. Yuan, Y. Jia, L. Zhuang, H. Zhang, X. Yan, G. Liu, Y. Zhao, J. Liu, S. Wei, L. Song, Q. Wu, B. Ge, L. Zhang, K. Wang, X. Wang, C. R. Chang, X. Yao, J. Am. Chem. Soc., 2022, 144, 2171 32. H. Wang, S. Yin, Y. Xu, X. Li, A. A. Alshehri, Y. Yamauchi, H. Xue, Y. V. Kaneti, L. Wang, J. Mater. Chem. A, 2018, 6, 8662 33. T. J. Wang, Y. C. Jiang, J. W. He, F. M. Li, Y. Ding, P. Chen, Y. Chen, Carbon Energy, 2022, 4, 283 34. X. Zhao, X. Li, D. Xiao, M. Gong, L. An, P. Gao, J. Yang, D. Wang, Appl. Catal. B: Environ, 2021, 295, 120280 35. J. Fan, J. Wu, X. Cui, L. Gu, Q. Zhang, F. Meng, B. H. Lei, D. J. Singh, W. Zheng, J. Am. Chem. Soc., 2020, 142, 3645 36. K. Zhu, J. Ma, L. Chen, F. Wu, X. Xu, M. Xu, W. Ye, Y. Wang, P. Gao, Y. Xiong, ACS Catal., 2022, 12, 4840 37. J. Liang, S. Li, Y. Chen, X. Liu, T. Wang, J. Han, S. Jiao, R. Cao, Q. Li, J. Mater. Chem. A, 2020, 8, 15665 38. J. Zhang, F. Lv, Z. Li, G. Jiang, M. Tan, M. Yuan, Q. Zhang, Y. Cao, H. Zheng, L. Zhang, C. Tang, W. Fu, C. Liu, K. Liu, L. Gu, J. Jiang, G. Zhang, S. Guo, Adv. Mater., 2022, 34, e2105276 39. X. Mu, J. Gu, F. Feng, Z. Xiao, C. Chen, S. Liu, S. Mu, Adv. Sci., 2021, 8, 2002341 40. Q. Mao, P. Wang, Z. Wang, Y. Xu, X. Li, L. Wang, H. Wang, Appl. Mater. Today, 2022, 26, 101400 41. C. Cao, Q. Xu, Q.-L. Zhu, Chem Catal., 2022, 2, 693 42. C. Tang, S.-Z. Qiao, Matter, 2019, 1, 1454 43. F. Lv, B. Huang, J. Feng, W. Zhang, K. Wang, N. Li, J. Zhou, P. Zhou, W. Yang, Y. Du, D. Su, S. Guo, Natl. Sci. Rev., 2021, 8, nwab019 44. M. Luo, Z. Zhao, Y. Zhang, Y. Sun, Y. Xing, F. Lv, Y. Yang, X. Zhang, S. wang, Y. Qin, J. Y. Ma, F. Lin, D. Su, G. Lu, S. Guo, Nature, 2019, 574, 81 45. Q. Yun, Q. Lu, C. Li, B. Chen, Q. Zhang, Q. He, Z. Hu, Z. Zhang, Y. Ge, N. Yang, J. Ge, Y. B. He, L. Gu, H. Zhang, ACS Nano, 2019, 13, 14329 46. L. Y. Zhang, F. Wang, S. Wang, H. Huang, X. Meng, Y. Ouyang, W. Yuan, C. X. Guo, C. M. Li, Adv. Funct. Mater., 2020, 30, 2003933 47. L. Shi, Q. Wang, Q. Ren, Q. Yang, D. Zhao, Y. Feng, H. Chen, Y. Wang, Small, 2022, 18, e2103665 48. B. Zhang, G. Zhao, B. Zhang, L. Xia, Y. Jiang, T. Ma, M. Gao, W. Sun, H. Pan, Adv. Mater., 2021, 33, e2105400 49. Y. Yan, H. Shan, G. Li, F. Xiao, Y. Jiang, Y. Yan, C. Jin, H. Zhang, J. Wu, D. Yang, Nano Lett., 2016, 16, 7999 50. Z. Zhang, Y. Liu, B. Chen, Y. Gong, L. Gu, Z. Fan, N. Yang, Z. Lai, Y. Chen, J. Wang, Y. Huang, M. Sindoro, W. Niu, B. Li, Y. Zong, Y. Yang, X. Huang, F. Huo, W. Huang, H. Zhang, Adv. Mater., 2016, 28, 10282 51. H. Yu, T. Zhou, Z. Wang, Y. Xu, X. Li, L. Wang, H. Wang, Angew. Chem. Int. Ed, 2021, 60, 12027 52. R. Wu, Y. Li, W. Gong, P. K. Shen, ACS Sustainable Chem. Eng., 2019, 7, 8419 53. H. Wang, S. Jiao, S. Liu, S. Wang, T. Zhou, Y. Xu, X. Li, Z. Wang, L. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 30479 54. M. Farsadrooh, J. Torrero, L. Pascual, M. A. Peña, M. Retuerto, S. Rojas, Appl. Catal. B: Environ., 2018, 237, 866 55. K. Deng, T. Zhou, Q. Mao, S. Wang, Z. Wang, Y. Xu, X. Li, H. Wang, L. Wang, Adv. Mater., 2022, 34, e2110680 56. B. Jiang, Y. Guo, J. Kim, A. E. Whitten, K. Wood, K. Kani, A. E. Rowan, J. Henzie, Y. Yamauchi, J. Am. Chem. Soc., 2018, 140, 12434 57. C. Tang, N. Zhang, Y. Ji, Q. Shao, Y. Li, X. Xiao, X. Huang, Nano Lett., 2019, 19, 1336 58. L. Zhai, X. She, L. Zhuang, Y. Li, R. Ding, X. Guo, Y. Zhang, Y. Zhu, K. Xu, H. J. Fan, S. P. Lau, Angew. Chem. Int. Ed., 2022, 61, e202116057 59. Z. Li, Y. Pei, R. Ma, Y. Wang, Y. Zhu, M. Yang, J. Wang, J. Mater. Chem. A, 2021, 9, 13109 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
-
Supplementary Information
Supporting_Information-2022-0005.R1 
Information
Article Metrics
-
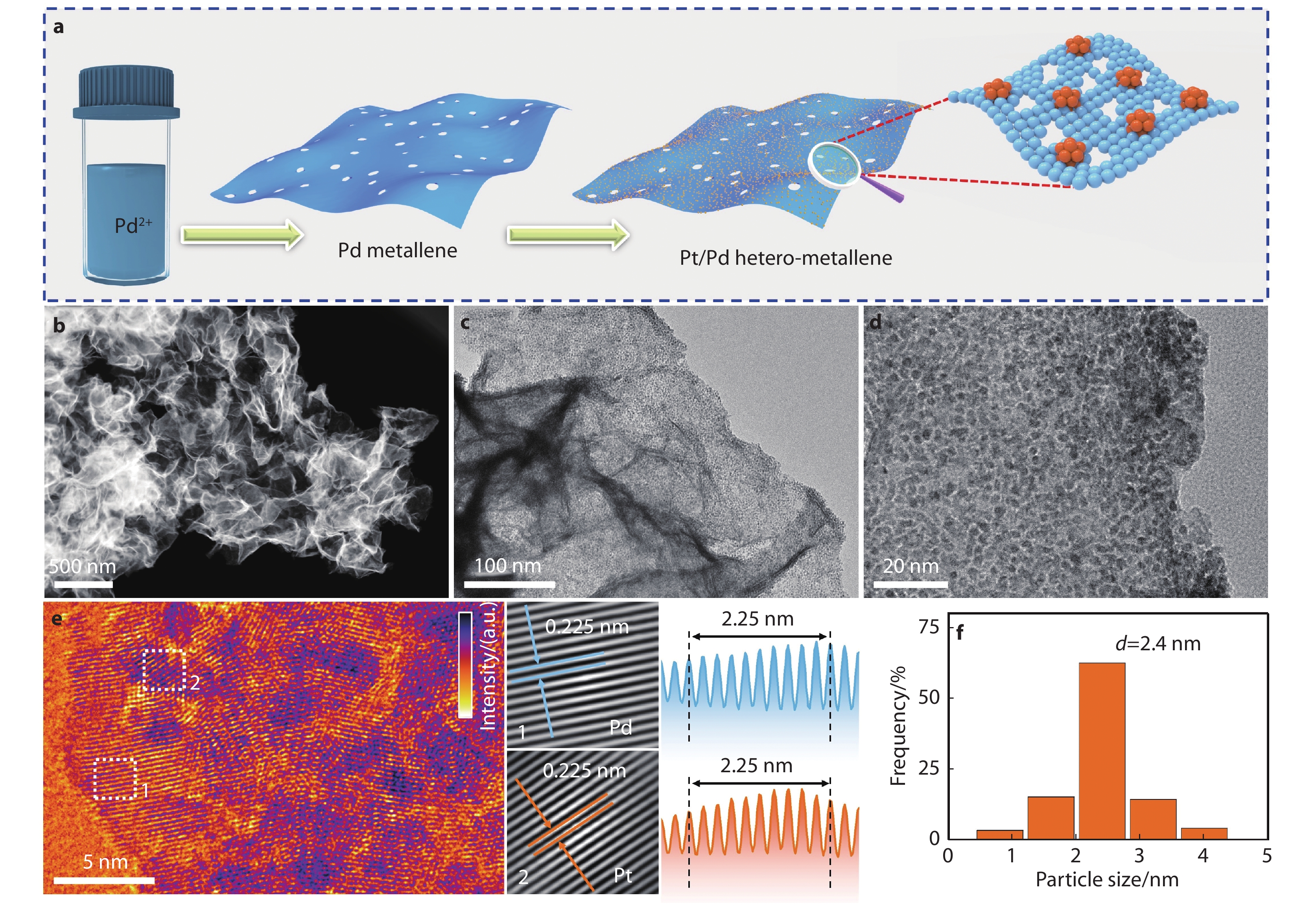
Figure 1.
a Schematic illustration for the synthesis of the Pt/Pd hetero-metallene. b HAADF-STEM and c and d TEM images of the Pt/Pd hetero-metallene. e False-color HRTEM image of the Pt/Pd hetero-metallene. f The particle size distribution of figure 1d. The insets in e display the lattice fringes and corresponding atomic absorption intensity profile of the square region.
-
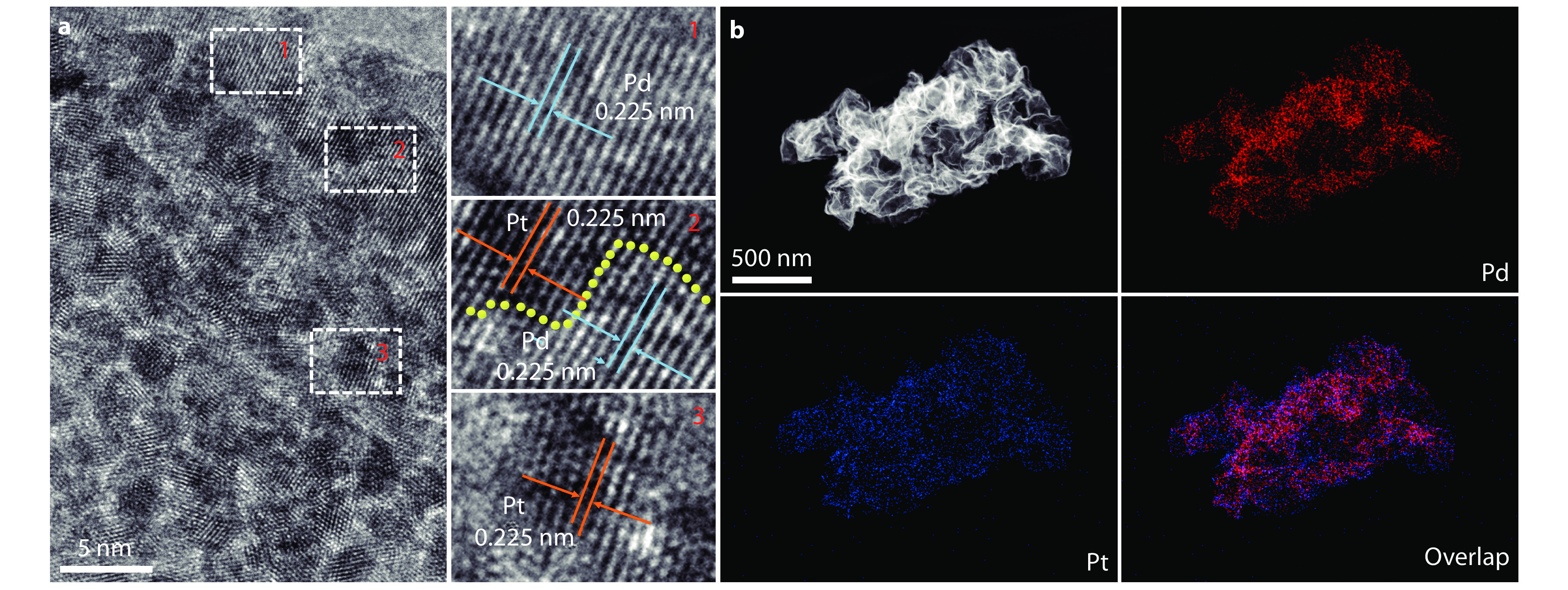
Figure 2.
a HRTEM image of the Pt/Pd hetero-metallene. b HAADF-STEM and EDS mapping images of the Pt/Pd hetero-metallene.
-
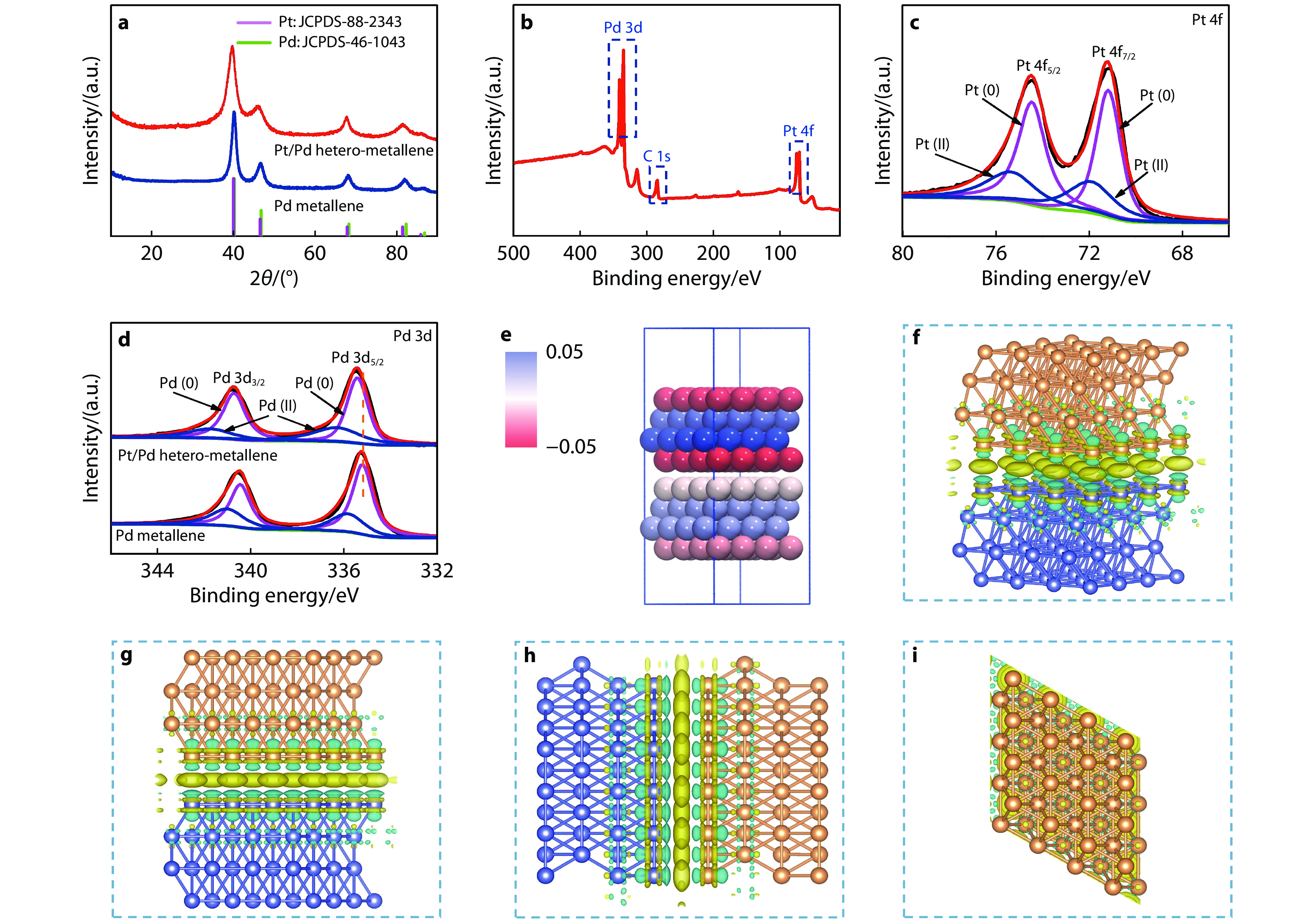
Figure 3.
a XRD patterns of the Pd metallene and Pt/Pd hetero-metallene. b XPS survey spectrum of the Pt/Pd hetero-metallene. c Pt 4f XPS spectra of the Pt/Pd hetero-metallene. d Pd 3d XPS spectra of Pt/Pd hetero-metallene and Pd metallene. e Bader charge of the Pt/Pd hetero-metallene. Negative and positive charge are indicated by red and blue balls, respectively. f Electron density difference on the Pt/Pd hetero-metallene. g, h and i Main side view, side view and top-view of electron density difference on the Pt/Pd hetero-metallene, respectively. The charge accumulation and depletion are indicated by cyan and yellow area, respectively. The isosurface level is 0.007 e Å−3.
-
Figure 4.
a Mass-normalized and b ECSA-normalized CVs of MOR for various catalysts recorded at a scan rate of 50 mV s−1 with 1 M KOH + 1 M CH3OH. c Comparison of mass activities and specific activities for various catalysts. d Chronoamperometric curves for various catalysts recorded at −0.2 V in 1 M KOH + 1 M CH3OH.
-
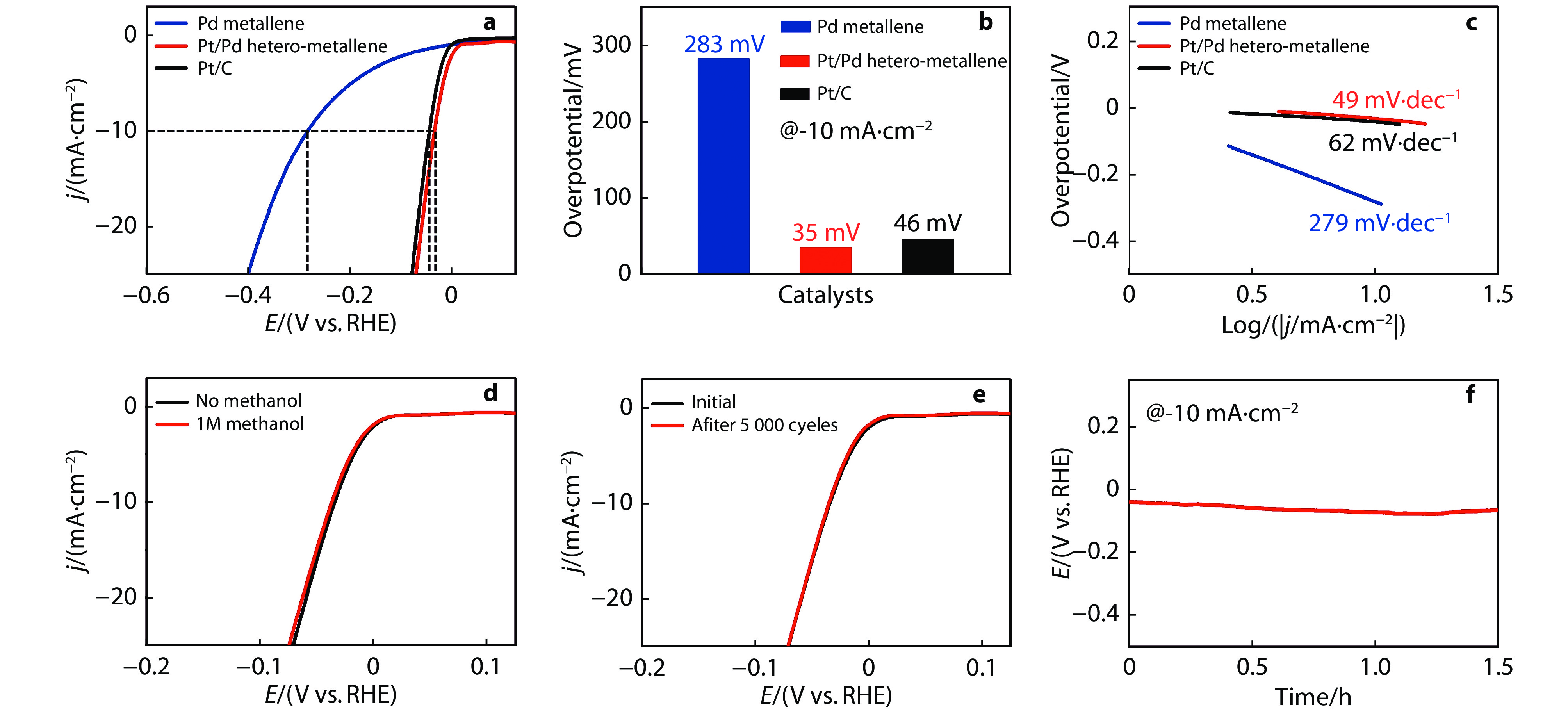
Figure 5.
a HER polarization curves for various catalysts in 1 M KOH and b the comparison of overpotentials at 10 mA cm−2. c Tafel slope plots for various catalysts. d HER polarization curves for the Pt/Pd hetero-metallene in 1 M KOH with and without 1 M CH3OH. e HER polarization curves for the Pt/Pd hetero-metallene before and after
5000 cycles in 1 M KOH. f Chronopotentiometry curves for the Pt/Pd hetero-metallene at a constant cathodic current density of 10 mA cm−2 in 1 M KOH for 15 h. -
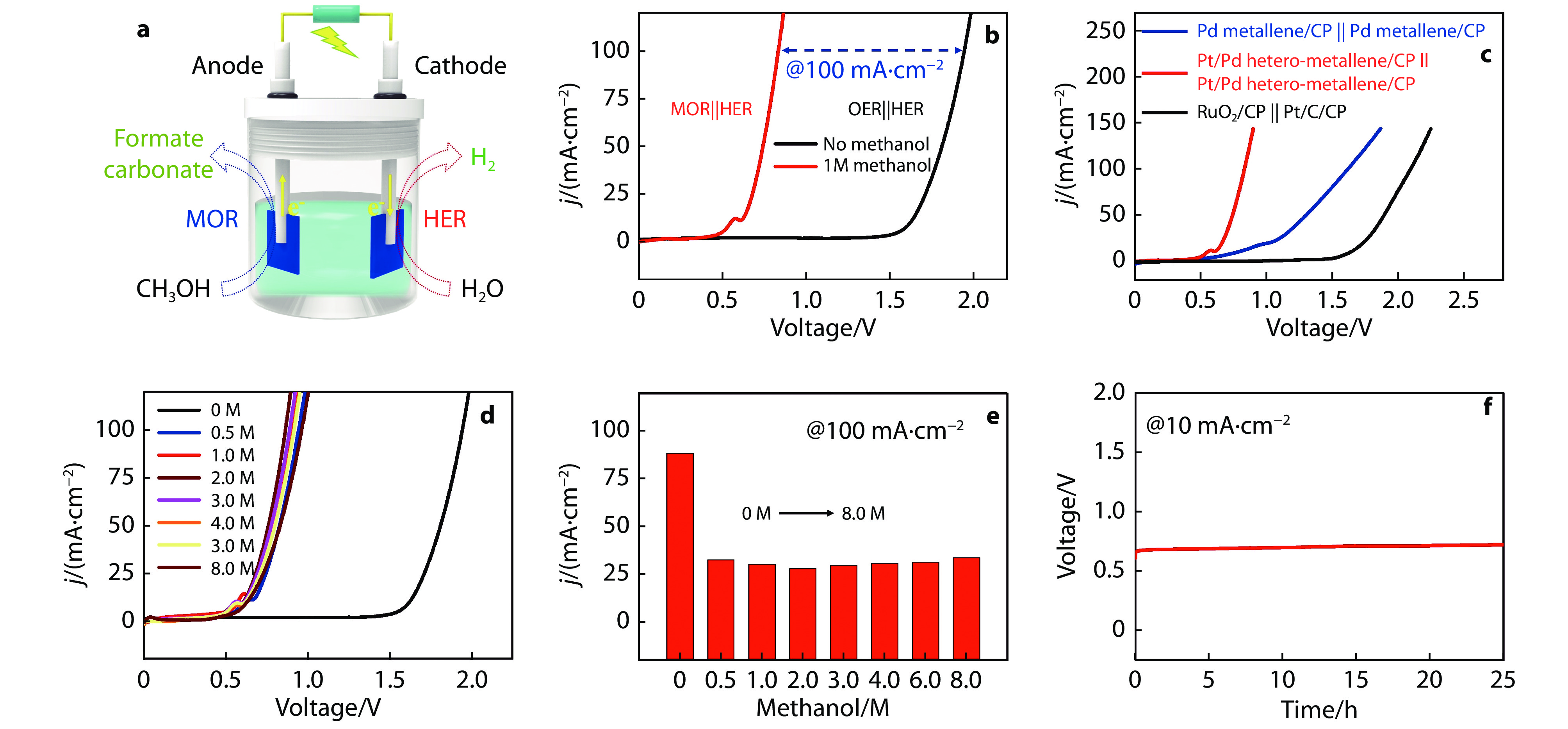
Figure 6.
a Schematic illustration for two-electrode CH3OH-assisted water splitting system. b LSV curves of the Pt/Pd hetero-metallene as anode and cathode in 1 M KOH with and without 1 M CH3OH. c LSV curves of various catalysts in 1 M KOH + 1 M CH3OH in a two-electrode system. d LSV curves of Pt/Pd hetero-metallene || Pt/Pd hetero-metallene in 1 M KOH with different CH3OH concentrations and e corresponding voltages at 100 mA cm−2. f Chronopotentiometry curves of Pt/Pd hetero-metallene || Pt/Pd hetero-metallene at a constant current density of 10 mA cm−2 in 1 M KOH + 1 M CH3OH for 25 h.

 DownLoad:
DownLoad: