| Citation: | Runyuan Cao, Yifan Yu, Chilin Li. Metal fluorides emerging as fast-charging and high-capacity cathodes from the viewpoint of open framework strategies[J]. Energy Lab, 2025, 3(1): 240017. doi: 10.54227/elab.20240017 |
Metal fluorides emerging as fast-charging and high-capacity cathodes from the viewpoint of open framework strategies
-
Abstract
The urgent demand for both large energy density and reliable safety has triggered broad-scale research in developing brand-new energy storage systems, including metal fluoride FeF3 as conversion-type cathode, in view of its high capacity and energy density based on the multi-electron redox principle. Its present development is plagued by the inferior ion conductivity resulting from the dense and compact original structure with limited ion diffusion channel, further leading to the huge volumetric expansion and slow reaction kinetics. Based on the performance degradation mechanism, it is crucial to manipulate the material structure from a molecule level with multidimensional transport channels, that is, an open framework strategy. Here, a timely review on the current situations for open framework and its application into fluoride-based cathode is presented. Specifically, the formation mechanisms for various open frameworks and the multiple functions of channel fillers are highlighted. We also focus on the construction of water-free open framework cathode and its combination with other hetero-doping, microstructure and electrolyte modulation strategies, in order to induce a more enduring cycling referring to high-capacity conversion reaction. With this review as background, new conception into open framework strategy and approach challenges are discussed, aiming at propelling the employment of metal fluoride cathodes to large-scale energy storage devices in the near future.
-
-
References
1. X. Fan, E. Hu, X. Ji, Y. Zhu, F. Han, S. Hwang, J. Liu, S. Bak, Z. Ma, T. Gao, S. C. Liou, J. Bai, X. Q. Yang, Y. Mo, K. Xu, D. Su and C. Wang, Nature Communications, 2018, 9, 2324 2. L. Li, F. Meng and S. Jin, Nano Letters, 2012, 12, 6030 3. K. Turcheniuk, D. Bondarev, V. Singhal and G. Yushin, Nature, 2018, 559, 467 4. T. Wu, Y. Cui, K. Wei, C. Lai, Y. Zhao, S. Ni, Y. Chen, X. Gao, Y. Cui and C. Li, Nano Energy, 2022, 103, 107843 5. A. W. Schäfer, S. R. Barrett, K. Doyme, L. M. Dray, A. R. Gnadt, R. Self, A. O’Sullivan, A. P. Synodinos and A. J. Torija, Nature Energy, 2019, 4, 160 6. D.-L. Ma, Z.-Y. Cao, H.-G. Wang, X.-L. Huang, L.-M. Wang and X.-B. Zhang, Energy Environmental Science, 2012, 5, 8538 7. W. Zhang, P. N. Duchesne, Z.-L. Gong, S.-Q. Wu, L. Ma, Z. Jiang, S. Zhang, P. Zhang, J.-X. Mi and Y. Yang, The Journal of Physical Chemistry C, 2013, 117, 11498 8. H. Li, P. Balaya and J. Maier, Journal of the Electrochemical Society, 2004, 151, A1878 9. C. Li, L. Gu, J. Tong, S. Tsukimoto and J. Maier, Advanced Functional Materials, 2011, 21, 1391 10. H. Wu, J. Hu, S. Yu and C. Li, Energy Environmental Science, 2025, 18, 923 11. C. Li, K. Chen, X. Zhou and J. Maier, npj Computational Materials, 2018, 4, 22 12. T. Li, L. Li, Y. L. Cao, X. P. Ai and H. X. Yang, The Journal of Physical Chemistry C, 2010, 114, 3190 13. S. W. Kim, D. H. Seo, H. Gwon, J. Kim and K. Kang, Advanced Materials, 2010, 22, 5260 14. K. Chen, W. Qiu, M. Lei, C. Lai, J. Liu and C. Li, Matter, 2024, 7, 3907 15. L. Guo, X. Gao, Q. Chen, H. Li, J. Ren, R. Wang, R. Shi, W. Gao and Y. Bai, Journal of Materials Chemistry A, 2024, 12, 32794 16. Z. Jiang, Y. Wang, X. Chen, F. Chu, X. Jiang, F. Kwofie, Q. Pei, S. Luo, J. Arbiol and F. Wu, Journal of Materials Chemistry A, 2023, 11, 21541 17. C. Li, L. Gu, S. Tsukimoto, P. A. van Aken and J. Maier, Advanced Materials, 2010, 22, 3650 18. C. Li, C. Yin, L. Gu, R. E. Dinnebier, X. Mu, P. A. van Aken and J. Maier, Journal of the American Chemical Society, 2013, 135, 11425 19. C. Li, L. Gu, J. Tong and J. Maier, ACS Nano, 2011, 5, 2930 20. C. Li, X. Mu, P. A. van Aken and J. Maier, Advanced Energy Materials, 2013, 3, 113 21. C. Li, C. Yin, X. Mu and J. Maier, Chemistry of Materials, 2013, 25, 962 22. J. Hu, Y. Zhang, D. Cao and C. Li, Journal of Materials Chemistry A, 2016, 4, 16166 23. Y. Han, J. Hu, C. Yin, Y. Zhang, J. Xie, D. Yin and C. Li, Journal of Materials Chemistry A, 2016, 4, 7382 24. Y. Han, M. Yang, Y. Zhang, J. Xie, D. Yin and C. Li, Chemistry of Materials, 2016, 28, 3139 25. D. Cao, C. Yin, D. Shi, Z. Fu, J. Zhang and C. Li, Advanced Functional Materials, 2017, 27, 1701130 26. W. Qiu, Z. Li, K. Chen, C. Li, J. Liu and W. Zhang, ACS Applied Materials & Interfaces, 2019, 11, 37768 27. D. S. Jacob, L. Bitton, J. Grinblat, I. Felner, Y. Koltypin and A. Gedanken, Chemistry of Materials, 2006, 18, 3162 28. N. Yamakawa, M. Jiang, B. Key and C. P. Grey, Journal of the American Chemical Society, 2009, 131, 10525 29. K. Lemoine, A. Wizner, S. Auguste, J.-M. Grenèche, H. Kojitani, M. Akaogi and Y. Inaguma, Open Ceramics, 2021, 6, 100123 30. K. Lemoine, A. Hémon-Ribaud, M. Leblanc, J. Lhoste, J.-M. Tarascon and V. Maisonneuve, Chemical Reviews, 2022, 122, 14405 31. G. Benner and R. Hoppe, Journal of Fluorine Chemistry, 1990, 46, 283 32. I. D. Gocheva, M. Nishijima, T. Doi, S. Okada, J.-i. Yamaki and T. Nishida, Journal of Power Sources, 2009, 187, 247 33. A. Martin, M. L. Doublet, E. Kemnitz and N. Pinna, Advanced Functional Materials, 2018, 28, 1802057 34. Y. Yamada, T. Doi, I. Tanaka, S. Okada and J.-i. Yamaki, Journal of Power Sources, 2011, 196, 4837 35. A. Kitajou, H. Komatsu, K. Chihara, I. D. Gocheva, S. Okada and J.-i. Yamaki, Journal of Power Sources, 2012, 198, 389 36. Y. Zheng, S. Jitto, J. Hwang, K. Matsumoto and R. Hagiwara, ACS Applied Energy Materials, 2022, 5, 14361 37. Z. Li, B. Wang, C. Li, J. Liu and W. Zhang, Journal of Materials Chemistry A, 2015, 3, 16222 38. K. Lemoine, L. Zhang, D. Dambournet, J.-M. Grenèche, A. Hémon-Ribaud, M. Leblanc, O. J. Borkiewicz, J.-M. Tarascon, V. Maisonneuve and J. Lhoste, Chemistry of Materials, 2019, 31, 4246 39. Y. Laligant, J. Pannetier, P. Labbe and G. Ferey, Journal of Solid State Chemistry, 1986, 62, 274 40. K. Lemoine, L. Zhang, J.-M. Grenèche, A. Hémon-Ribaud, M. Leblanc, A. Guiet, C. Galven, J.-M. Tarascon, V. Maisonneuve and J. r. m. Lhoste, The Journal of Physical Chemistry C, 2019, 123, 21386 41. K. Lemoine, R. Moury, J. Lhoste, A. Hémon-Ribaud, M. Leblanc, J.-M. Grenèche, J.-M. Tarascon and V. Maisonneuve, Dalton Transactions, 2020, 49, 8186 42. J. L. Hu, K. Y. Chen, Z. G. Yao and C. L. Li, Science Bulletin, 2021, 66, 694 43. N. Louvain, A. Fakhry, P. Bonnet, M. El-Ghozzi, K. Guérin, M.-T. Sougrati, J.-C. Jumas and P. Willmann, CrystEngComm, 2013, 15, 3664 44. K. Lemoine, R. Moury, E. Durand, E. A.-d. Dompablo, E. Moran, M. Leblanc, A. Hemon-Ribaud, J.-M. Greneche, C. Galven and V. Gunes, Crystal Growth Design, 2021, 21, 935 45. K. Chen, M. Lei, Z. Yao, Y. Zheng, J. Hu, C. Lai and C. Li, Science Advances, 2021, 7, eabj1491 46. C. Li, L. Gu and J. Maier, Advanced Functional Materials, 2012, 22, 1145 47. F. Badway, F. Cosandey, N. Pereira and G. Amatucci, Journal of The Electrochemical Society, 2003, 150, A1318 48. R. E. Doe, K. A. Persson, Y. S. Meng and G. Ceder, Chemistry of Materials, 2008, 20, 5274 49. Y. Y. Liu, J. W. Meng, M. Lei, Y. F. Yu, C. Z. Lai and C. L. Li, Advanced Functional Materials, 2023, 33, 2208013 50. L. Li, R. Jacobs, P. Gao, L. Gan, F. Wang, D. Morgan and S. Jin, Journal of the American Chemical Society, 2016, 138, 2838 51. C. Lai, K. Chen, Y. Zheng, J. Meng, J. Hu and C. Li, Journal of Energy Chemistry, 2023, 78, 178 52. S. Sun, Z. Han, W. Liu, Q. Xia, L. Xue, X. Lei, T. Zhai, D. Su and H. Xia, Nature Communications, 2023, 14, 6662 53. R. R. Li, J. He, M. Lei, M. H. Yang and C. L. Li, Chemical Engineering Journal, 2022, 446, 137294 54. K. Karki, L. Wu, Y. Ma, M. J. Armstrong, J. D. Holmes, S. H. Garofalini, Y. Zhu, E. A. Stach and F. Wang, Journal of the American Chemical Society, 2018, 140, 17915 55. A. W. Xiao, H. J. Lee, I. Capone, A. Robertson, T.-U. Wi, J. Fawdon, S. Wheeler, H.-W. Lee, N. Grobert and M. Pasta, Nature Materials, 2020, 19, 644 56. C. Lai, K. Chen, M. Lei, J. Hu, S. Chen and C. Li, Advanced Functional Materials, 2024, 34, 2312415 57. Q. Huang, K. Turcheniuk, X. Ren, A. Magasinski, D. Gordon, N. Bensalah and G. Yushin, Advanced Energy Materials, 2019, 9, 1803323 58. W. Gu, O. Borodin, B. Zdyrko, H.-T. Lin, H. Kim, N. Nitta, J. Huang, A. Magasinski, Z. Milicev, G. Berdichevsky and G. Yushin, Advanced Functional Materials, 2016, 26, 1507 59. O. Borodin, J. Self, K. A. Persson, C. Wang and K. Xu, Joule, 2020, 4, 69 60. Q. Huang, K. Turcheniuk, X. Ren, A. Magasinski, A.-Y. Song, Y. Xiao, D. Kim and G. Yushin, Nature Materials, 2019, 18, 1343 61. Y. Zhang, J. Meng, K. Chen, H. Wu, J. Hu and C. Li, ACS Energy Letters, 2020, 5, 1167 62. Y. Yu, C. Lai, M. Lei, K. Chen and C. Li, Materials Horizons, 2024, 11, 2169 63. C. Patel, E. Andre-Joyaux, J. A. Leitch, X. M. de Irujo-Labalde, F. Ibba, J. Struijs, M. A. Ellwanger, R. Paton, D. L. Browne, G. Pupo, S. Aldridge, M. A. Hayward and V. Gouverneur, Science, 2023, 381, 302 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
An IL-based mild fluorination strategy for open framework structures. a A schematic illustration of mild fluorination mechanism to prepare open framework fluorides.[17] Copyright 2010, Wiley. b A schematic illustration of interaction of IL with SWCNTs and photo of reaction product in IL. c A scheme that IL serves as an in-situ binder for pyrochlore fluoride grains.[19] Copyright 2011, American Chemical Society.
-
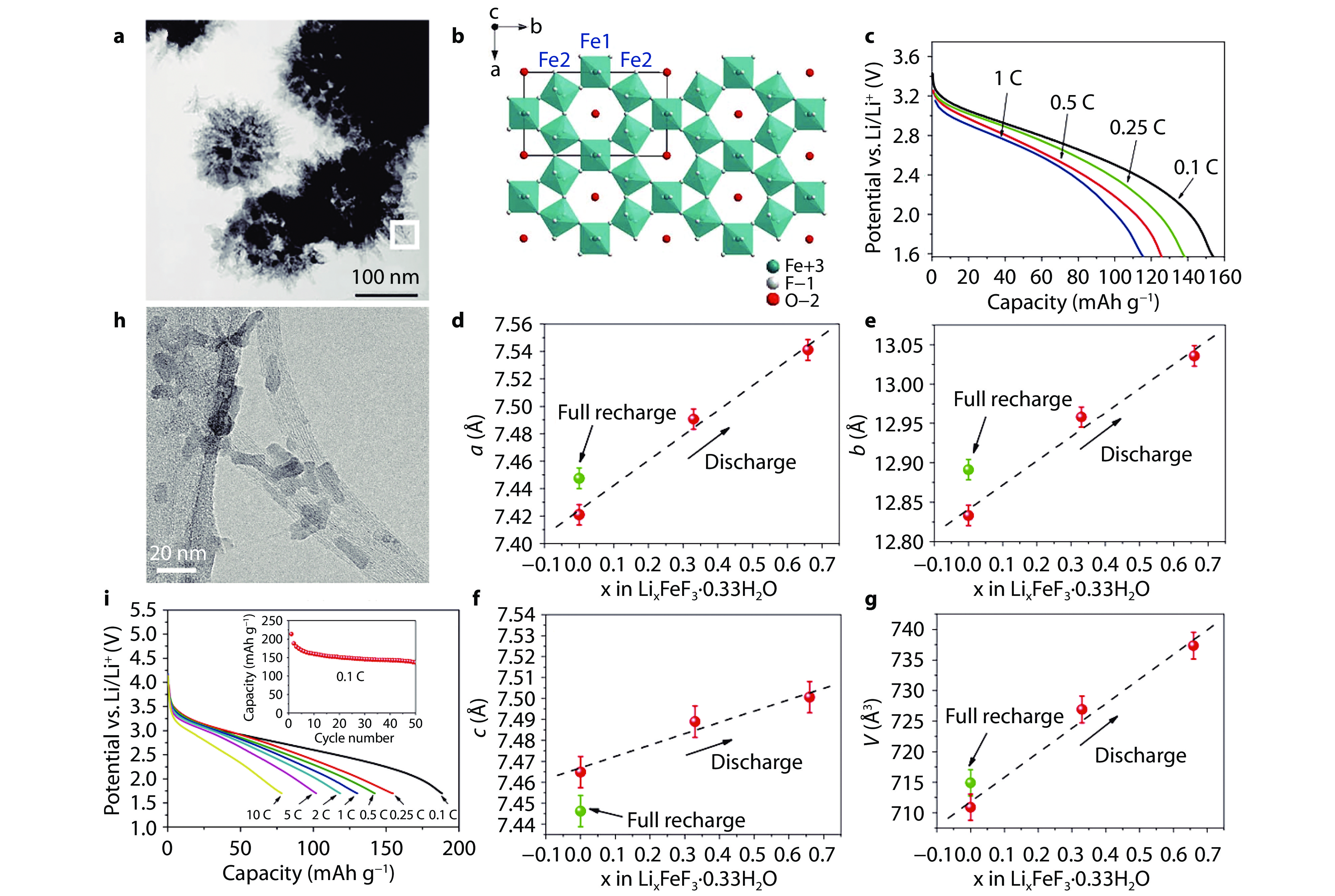
Figure 2.
Morphology, structure and performance of HTB-FeF3·0.33H2O-based cathode. a TEM image of HTB-FeF3·0.33H2O grains.[17] Copyright 2010, Wiley. b Structure view of FeF3·0.33H2O along [001] direction. c Discharge curves of HTB-FeF3·0.33H2O at various rates. Evolution of lattice parameters of HTB-FeF3·0.33H2O at various lithiation stages: lattice constant d a, e b, f c and g cell volume.[9] Copyright 2011, Wiley. h TEM image of SWCNTs-FeF3·0.33H2O. i Discharge curves of SWCNTs-FeF3·0.33H2O at various rates. Inset: cycling performance of SWCNTs-FeF3·0.33H2O at 0.1 C.[19] Copyright 2011, American Chemical Society.
-
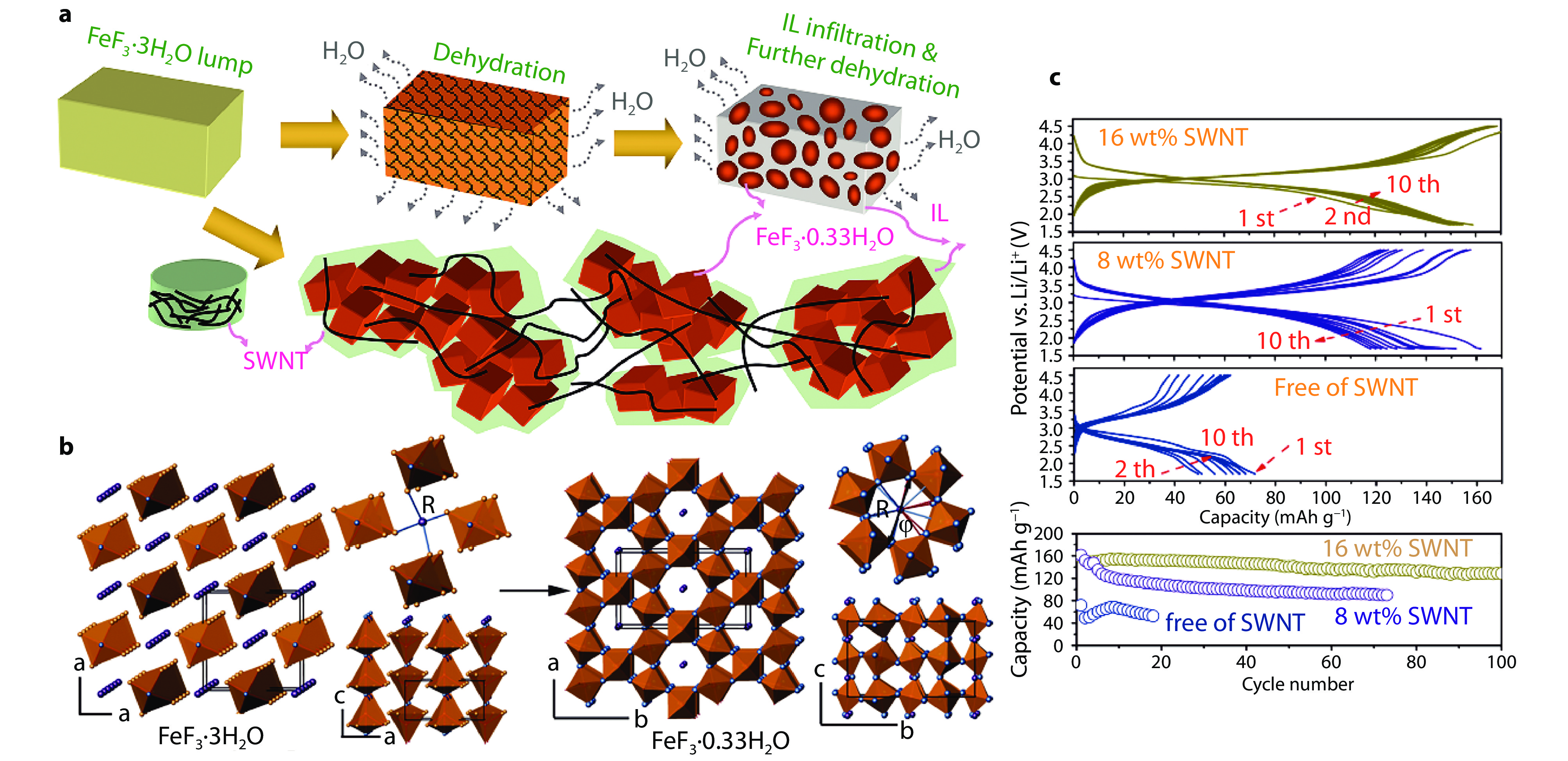
Figure 3.
Fabrication process, structure and electrochemical performance of HTB-FeF3·0.33H2O synthesized by top-down strategy. a A scheme of top-down synthesis process. b Evolution of crystallographic structures from FeF3·3H2O precursor to FeF3·0.33H2O product during top-down synthesis. The colors of O, F, Fe, and randomly occupied F/O are noted as purple, blue, red, and yellow, respectively. c Cycling performance of FeF3·0.33H2O cathodes with various contents of SWCNTs-wiring at 0.1 C. The voltage range is from 1.7 to 4.5 V.[21] Copyright 2013, American Chemical Society.
-
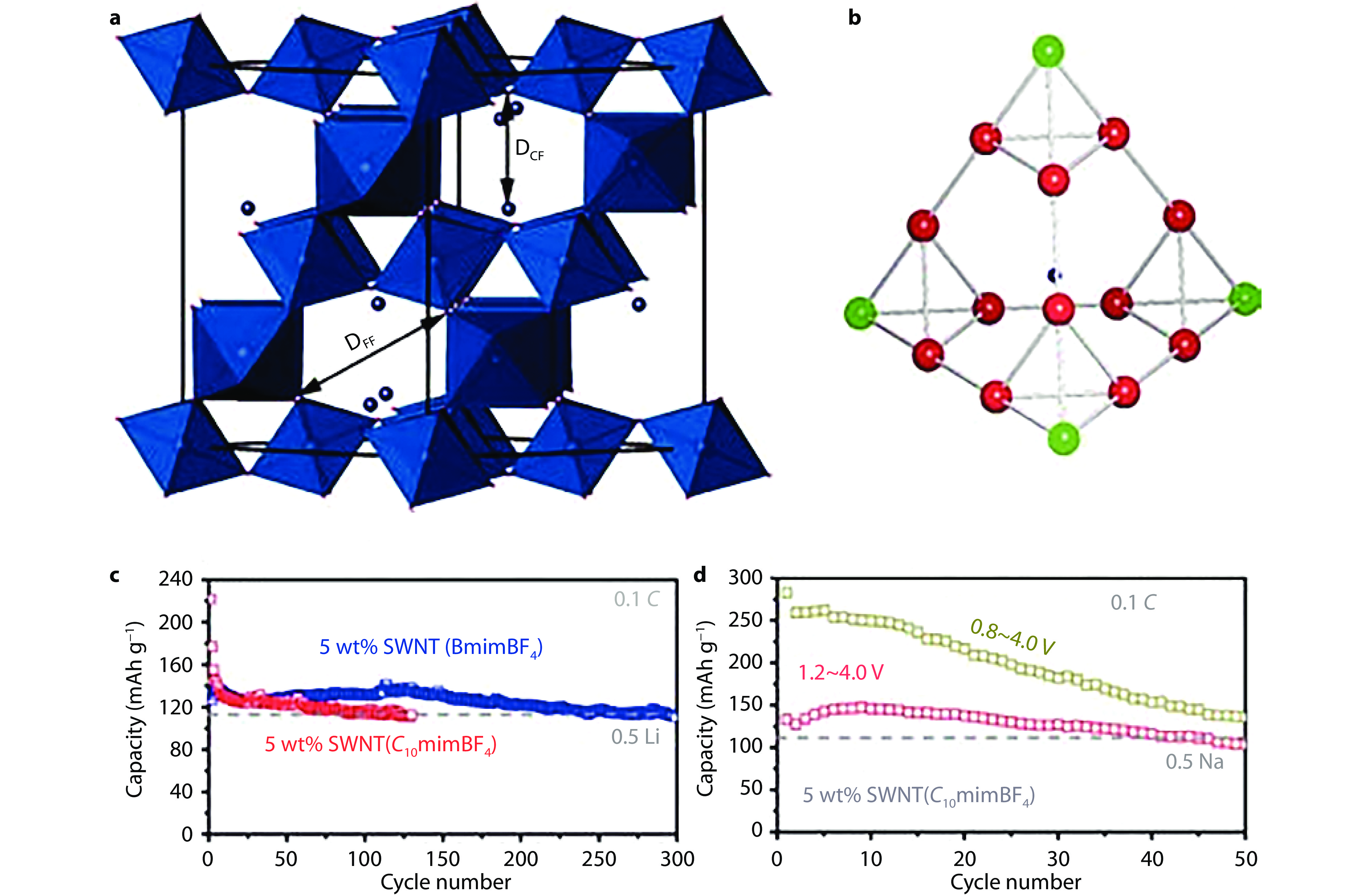
Figure 4.
Structure and electrochemistry of pyrochlore-type FeF3·0.5H2O. a Structure of pyrochlore-type FeF3·0.5H2O. b (FeF6)16 supertetrahedron with truncated tetrahedral cavity. The central Fe atoms are marked as red or green and the trapped O is marked as blue. c Cycling performance of FeF3·0.5H2O prepared from different ILs for Li storage at 0.1 C. d Cycling performance of FeF3·0.5H2O prepared from C10mimBF4 in different voltage ranges for Na storage at 0.1 C.[18] Copyright 2013, American Chemical Society.
-
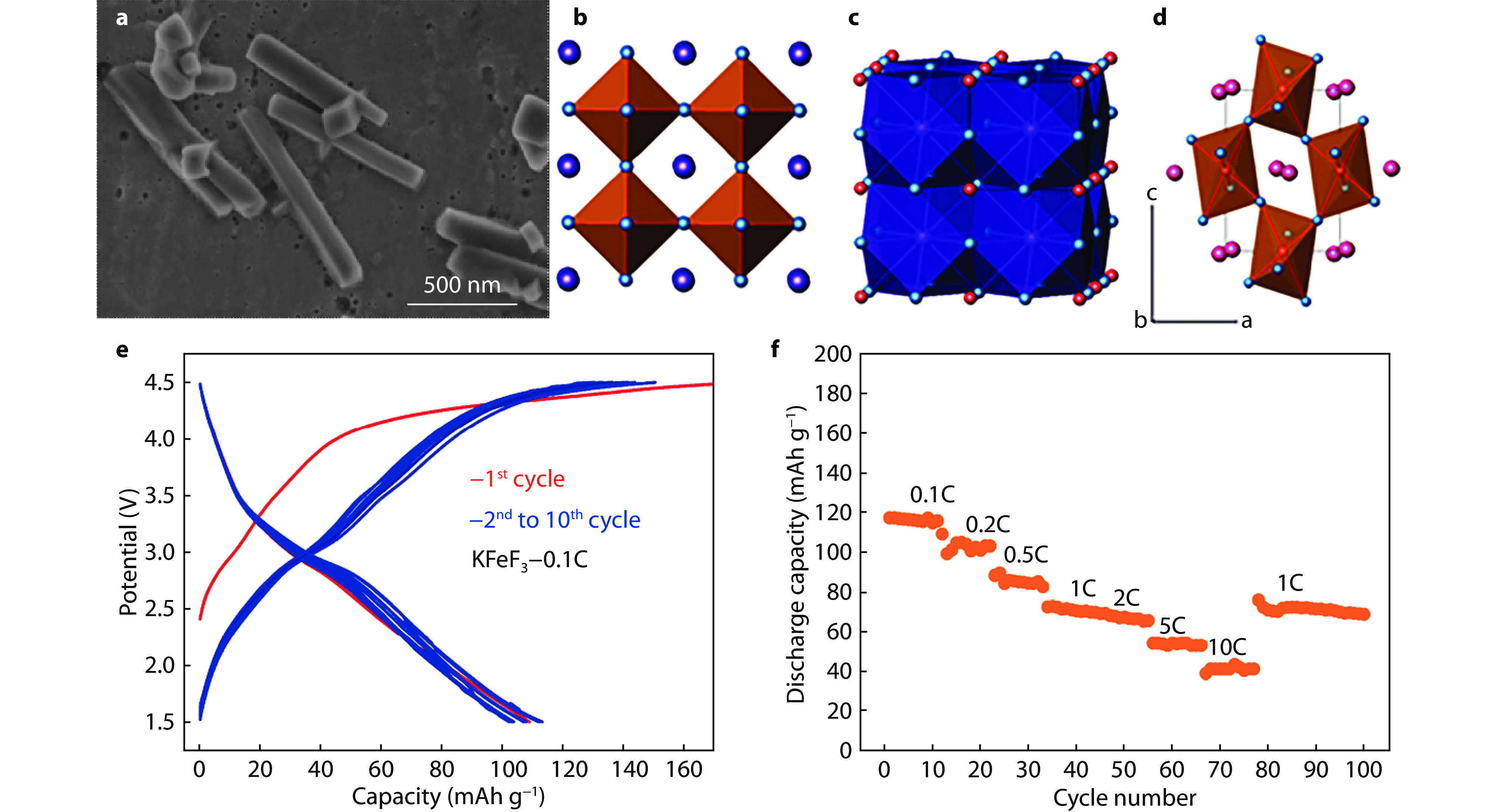
Figure 5.
K+-stuffing open framework structures and Na-storage capacity. a Synthesis schemes of TTB-type KxFeF3 and cubic perovskite KFeF3. b Structure view of TTB-type K0.6FeF3 along [001] direction. c Charge and discharge curves of K0.6FeF3-carbon nanocomposite in a voltage range of 1.5-4.5 V.[23] Copyright 2016, Royal Society of Chemistry.
-
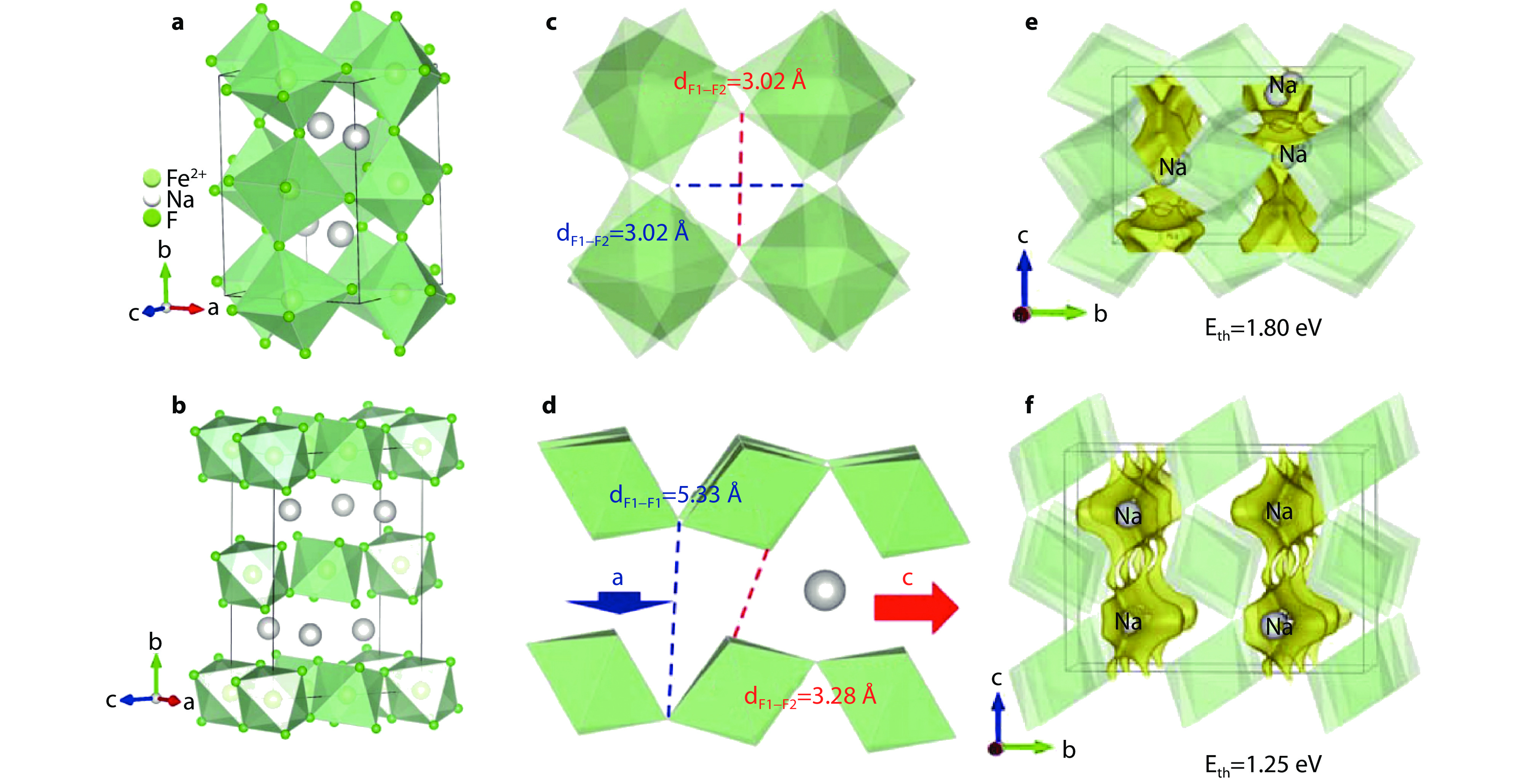
Figure 6.
Comparison of perovskite-NaFeF3 and post-perovskite-NaFeF3 open frameworks. Crystal structures of a perovskite-NaFeF3 and b post-perovskite-NaFeF3. c Illustration of F1-F2 distances in perovskite-NaFeF3 structure. d Illustration of F1-F1 and F1-F2 interlayer distances in post-perovskite-NaFeF3 structure. Calculated BVEL maps and Na+ diffusion thresholds along a axis for e perovskite-NaFeF3 and f post-perovskite-NaFeF3.[29] Copyright 2021, Elsevier.
-
Figure 7.
Morphology, structure and electrochemistry of cubic perovskite open framework. a SEM image of KFeF3. b FeF6 octahedra connecting with each other via vertex F− with K, Fe and F atoms marked as purple, red and blue. c Truncated-octahedra-like cavities in KFeF3 with K+-occupying centers. d Structure of NaFeF3 with Na, Fe and F atoms marked as pink, red and blue. The distortion of FeF6 octahedra is observed. e Cycling performance and voltage profiles of KFeF3-carbon during the first ten cycles. f Rate performance of KFeF3-carbon cathode.[25] Copyright 2017, Wiley.
-
Figure 8.
Effects of channel water in FeF3·nH2O. a Relative energies of FeF3 (in black) and relative formation energies of FeF3·nH2O (in red) as a function of torsion angle φ1. b Definition of torsion angles (φ1 and φ2) in anhydrous FeF3 with open framework. c Formation energies and structure evolution at various lithiation stages for FeF3·0.33H2O.[37] Copyright 2015, Royal Society of Chemistry.
-
Figure 9.
Dehydrated HTB-FeF3 prepared by ionothermal fluorination. a Scheme of ionothermal fluorination based on C10mimBF4. SEM images of b HTB-FeF3 and c HTB2 (i.e. HTB phase prepared in BmimBF4 reaction medium). d XRD patterns of different samples, HTB3 is a HTB phase prepared by a top-down strategy in C10mimBF4 reaction medium. e O1s XPS spectra for HTB3 (purple), HTB-FeF3 (green) and HTB2 (orange). f Intercalation electrochemical behavior and g conversion electrochemical behavior of HTB-FeF3.[22] Copyright 2016, Royal Society of Chemistry.
-
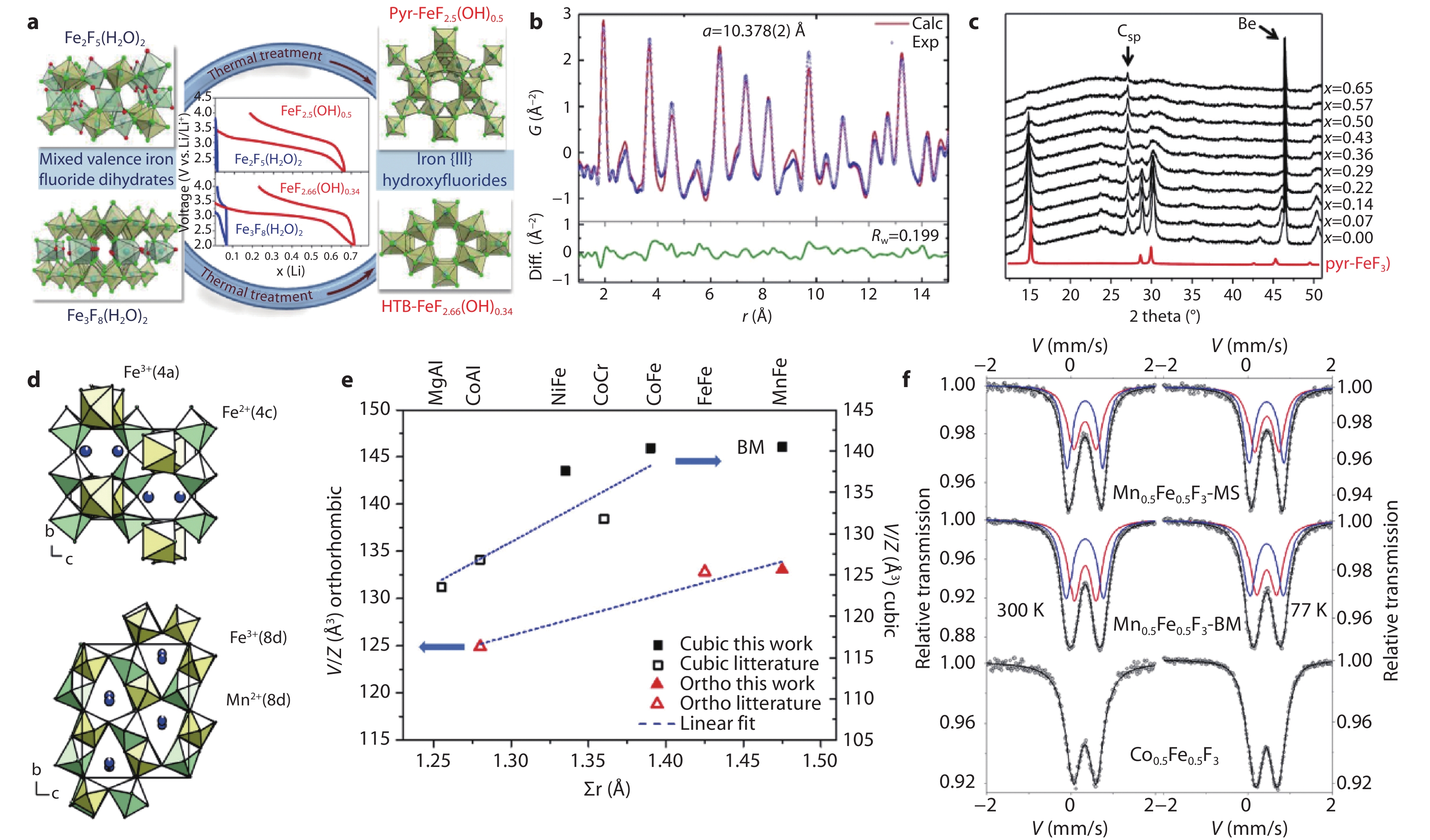
Figure 10.
Structure evolution of open framework iron fluorides via thermal treatment. a Scheme of thermal treatment method to prepare pyrochlore and HTB fluorides from open framework type precursors with massive hydration water. b PDF refinement on the product after thermal treatment of Fe2F5(H2O)2 at 200 °C (1 h). c In-situ XRD of pyrochlore-like FeF2.5(OH)0.5 during the first discharge.[38] Copyright 2019, American Chemical Society. d Crystal structures of (NH4)Fe2+Fe3+F6 along [100] direction (above) and (NH4)Mn2+Fe3+F6 along [100] direction (below). e Cell volume evolution of ammonium metal fluoride precursors. f 57Fe Mossbauer spectra for Mn0.5Fe0.5F3 at 300 and 77 K.[44] Copyright 2020, American Chemical Society.
-
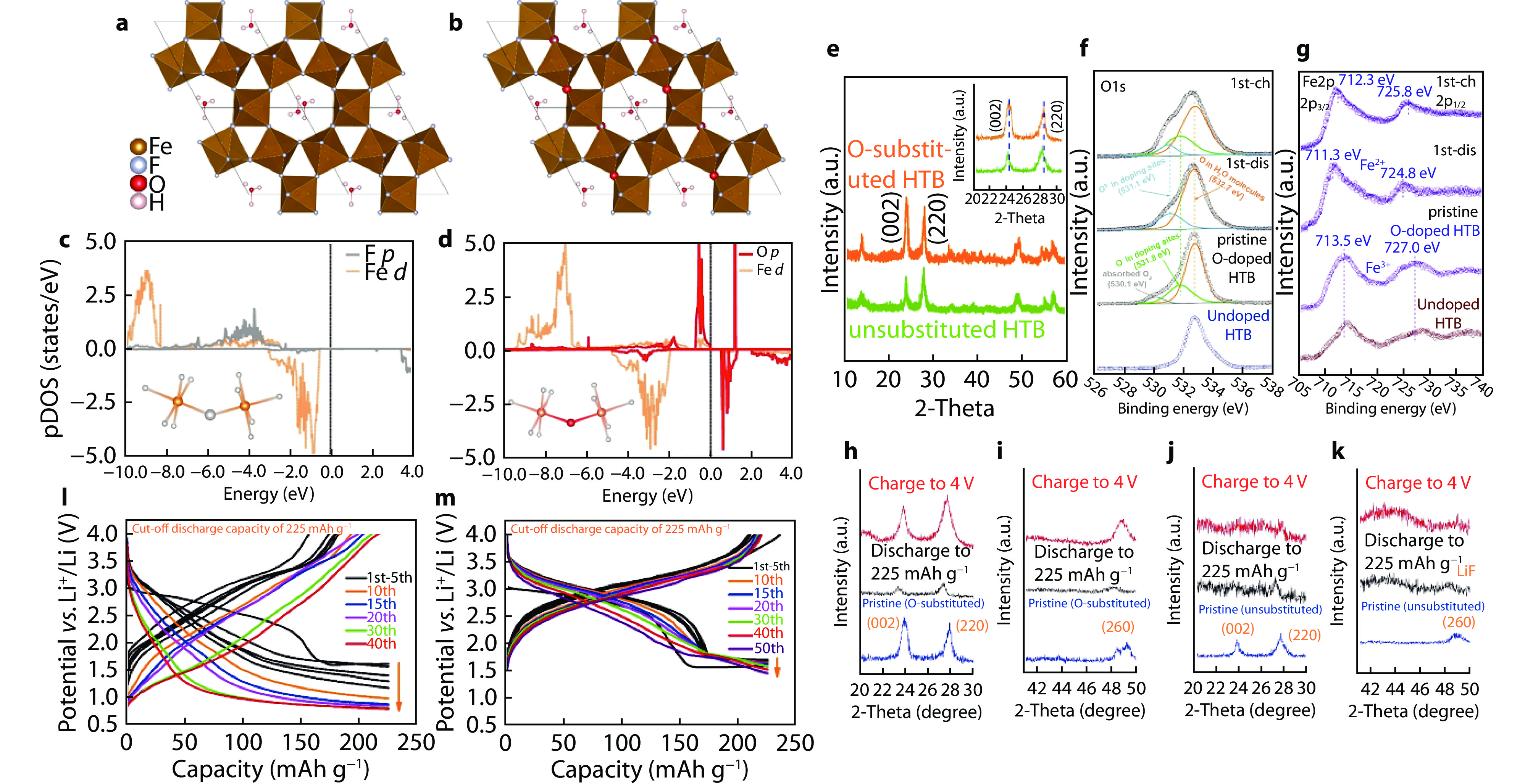
Figure 11.
Comparisons of characterization and electrochemistry between O-substituted and unsubstituted HTB phases. Structures of a unsubstituted FeF3·0.33H2O and b O-substituted FeF2.67O0.33·0.33H2O. pDOS of 2p orbitals of ligand anions (F− or O2−) in c FeF3·0.33H2O and d FeF2.67O0.33·0.33H2O. e XRD patterns of HTB phases with and without O substitution. Inset: magnified peaks in 20°-30°. f O1s and g Fe2p XPS spectra of substituted phase at various cycling states and pristine unsubstituted phase. XRD patterns of h,i substituted phase and j,k unsubstituted phase at various cycling states. Cycling performance and voltage profiles of l unsubstituted phase and m substituted phase with a cut-off discharge capacity of 225 mAh g−1.[26] Copyright 2019, American Chemical Society.
-
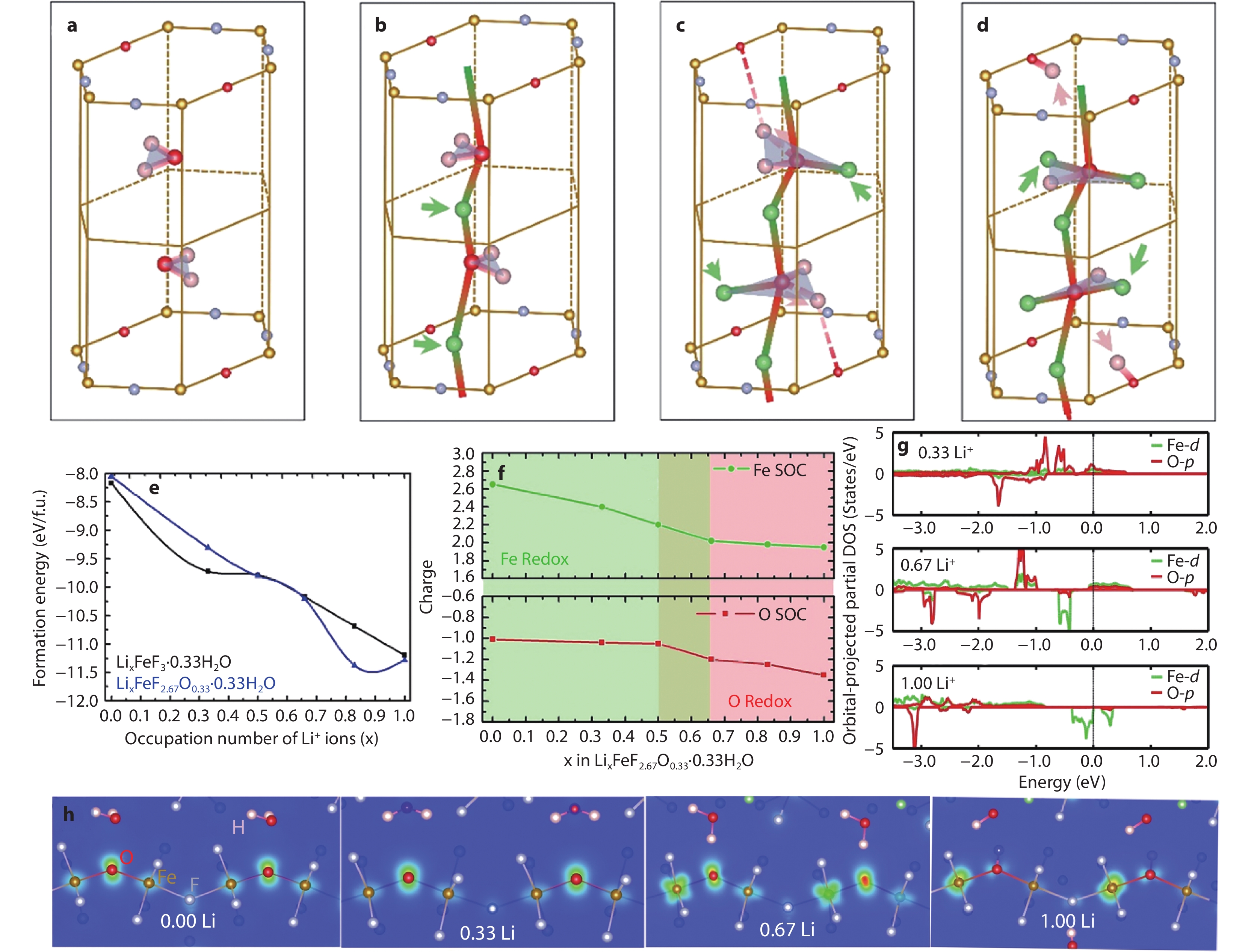
Figure 12.
Stabilization mechanism of FeF2.67O0.33·0.33H2O with low-coordinated O during discharge. Evolution of Li+ occupancy sites and hydrogen transfer in lithiated substituted phase (LixFeF2.67O0.33·0.33H2O) when x is a 0.00, b 0.33, c 0.67 and d 1.00 respectively, shadows are the H2O planes with the substitution by some Li+ ions, and arrows stand for Li+ insertion and hydrogen transfer. e Formation energy evolution of LixFeF3·0.33H2O and LixFeF2.67O0.33·0.33H2O. f Charge state evolution of Fe and substituted O during lithiation. g pDOSs of Fe and substituted O. h Charge density evolution of LixFeF2.67O0.33·0.33H2O at various lithiation stages.[26] Copyright 2019, American Chemical Society.
-
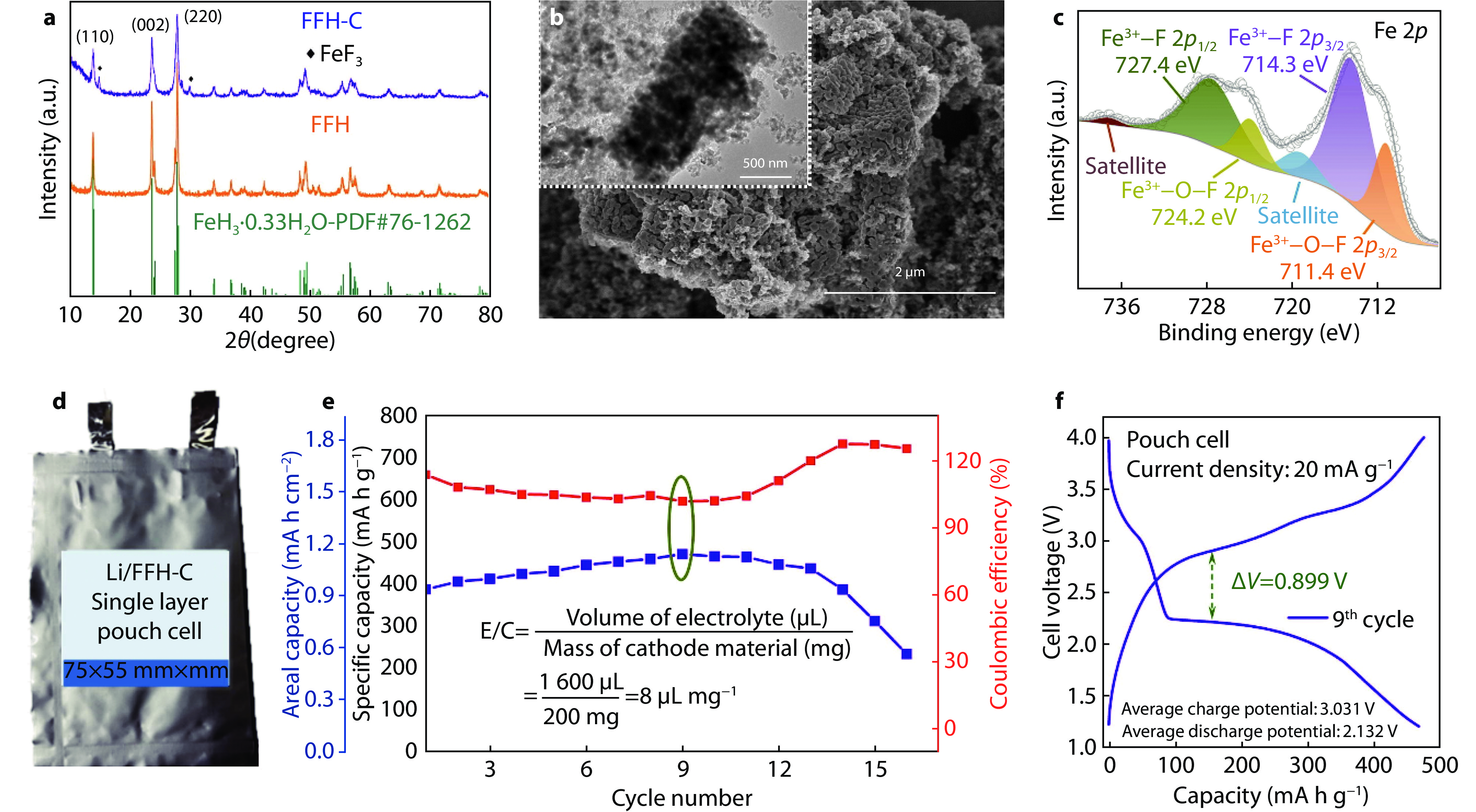
Figure 13.
Morphology, microstructure and performance of oxygen-doped HTB-FeF3 cathode. a XRD patterns of synthesized HTB-FeF3 with/without the assistance of Co2+ in precursor. b SEM and TEM images of oxygen-doped HTB-FeF3 showing a porous structure. c Fe 2p XPS spectrum of oxygen-doped HTB-FeF3. d Schematic of Li-HTB-FeF3 pouch cell. e Cycling performance of Li-HTB-FeF3 pouch cell at 20 mA g−1. f Galvanostatic charge-discharge curves of Li-HTB-FeF3 pouch cell in the 9th cycle.[51] Copyright 2023, Elsevier.
-
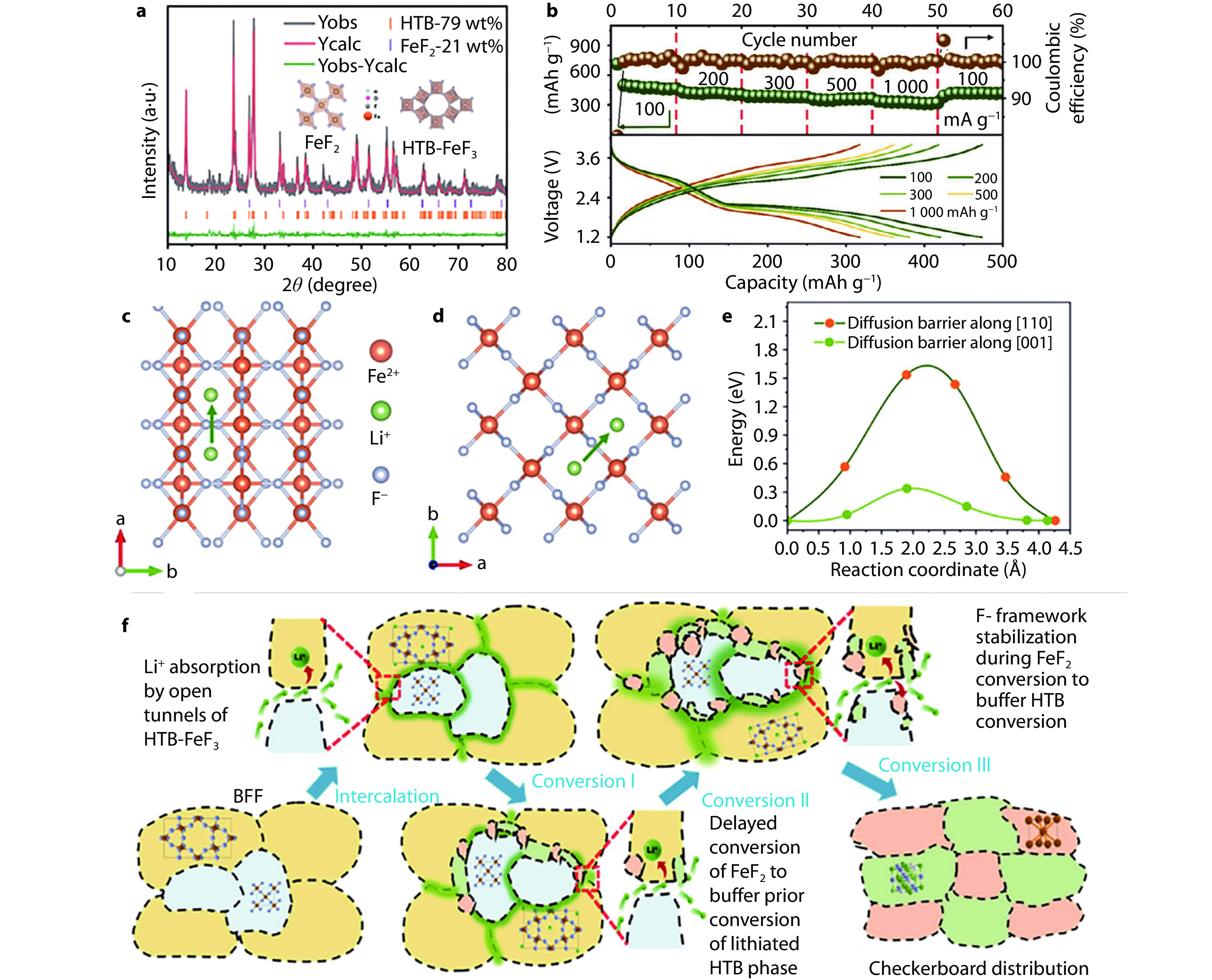
Figure 14.
Structure, performance and DFT calculation on heterostructure of HTB-FeF3 and FeF2. a XRD pattern and its Rietveld refinement of heterostructure cathode. b Rate performance and corresponding charge and discharge curves of heterostructure cathode. Top view of Li+ diffusion along c [001] and d [110] directions in FeF2 crystal structure. e Calculated Li+ diffusion barriers based on [001] and [110] directions. f Schematics of intercalation and conversion reactions of heterostructure containing HTB-FeF3 and FeF2.[56] Copyright 2024, Wiley.
-
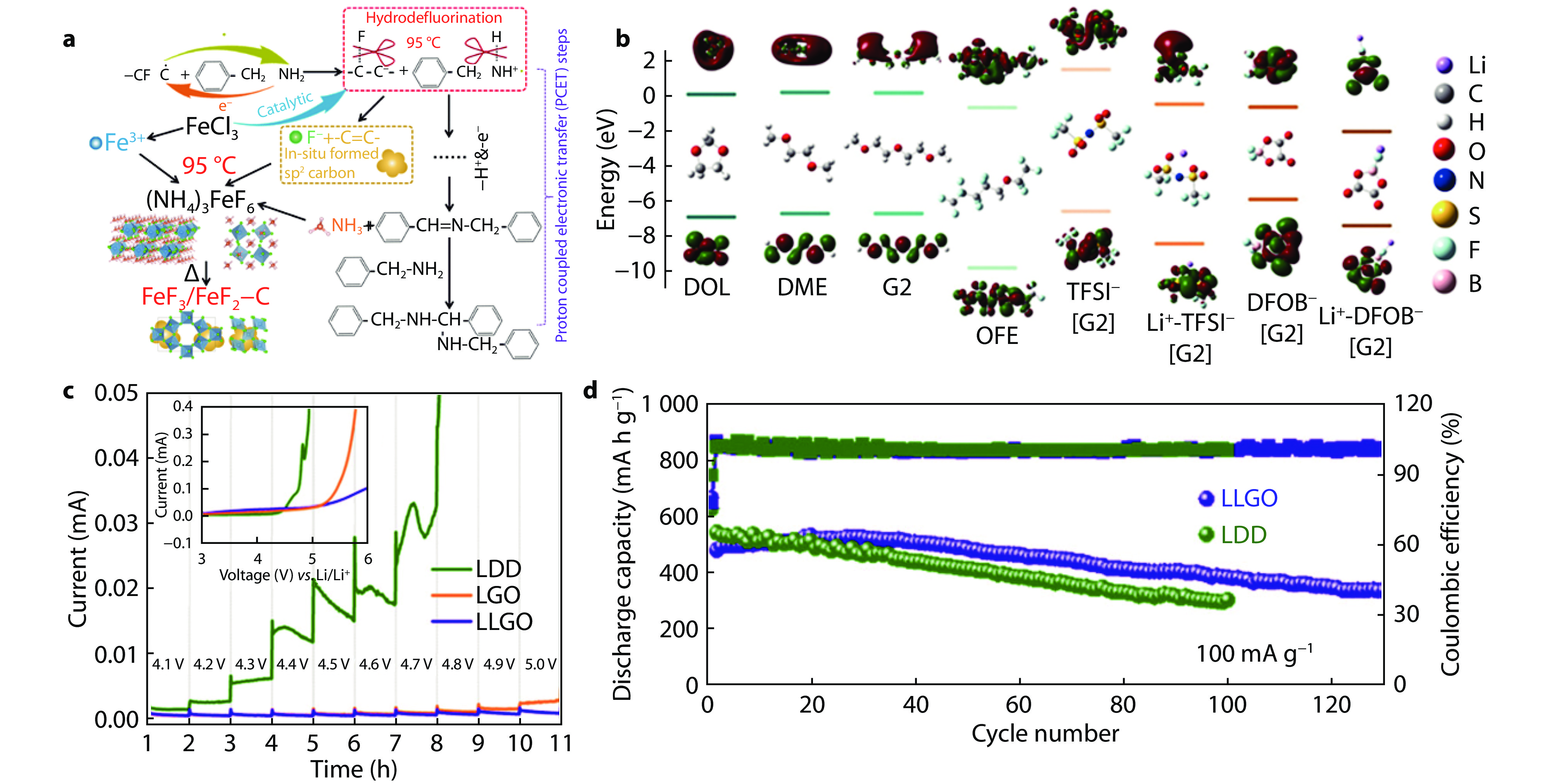
Figure 15.
Iron-based open framework fluoride synthesized by C-F mild scissoring fluorination and its coupling with LHCE electrolyte. a Schematic illustration of synthesis steps based on C-F scissoring method. b Calculated HOMO-LUMO energy levels of various solvent molecules, anions and Li+-anion pairs. c Oxidation stability and linear sweep voltammetry curves based on different electrolytes. d Cycling performance of FeF3 cathodes in different electrolytes.[62] Copyright 2024, Royal Society of Chemistry.
-
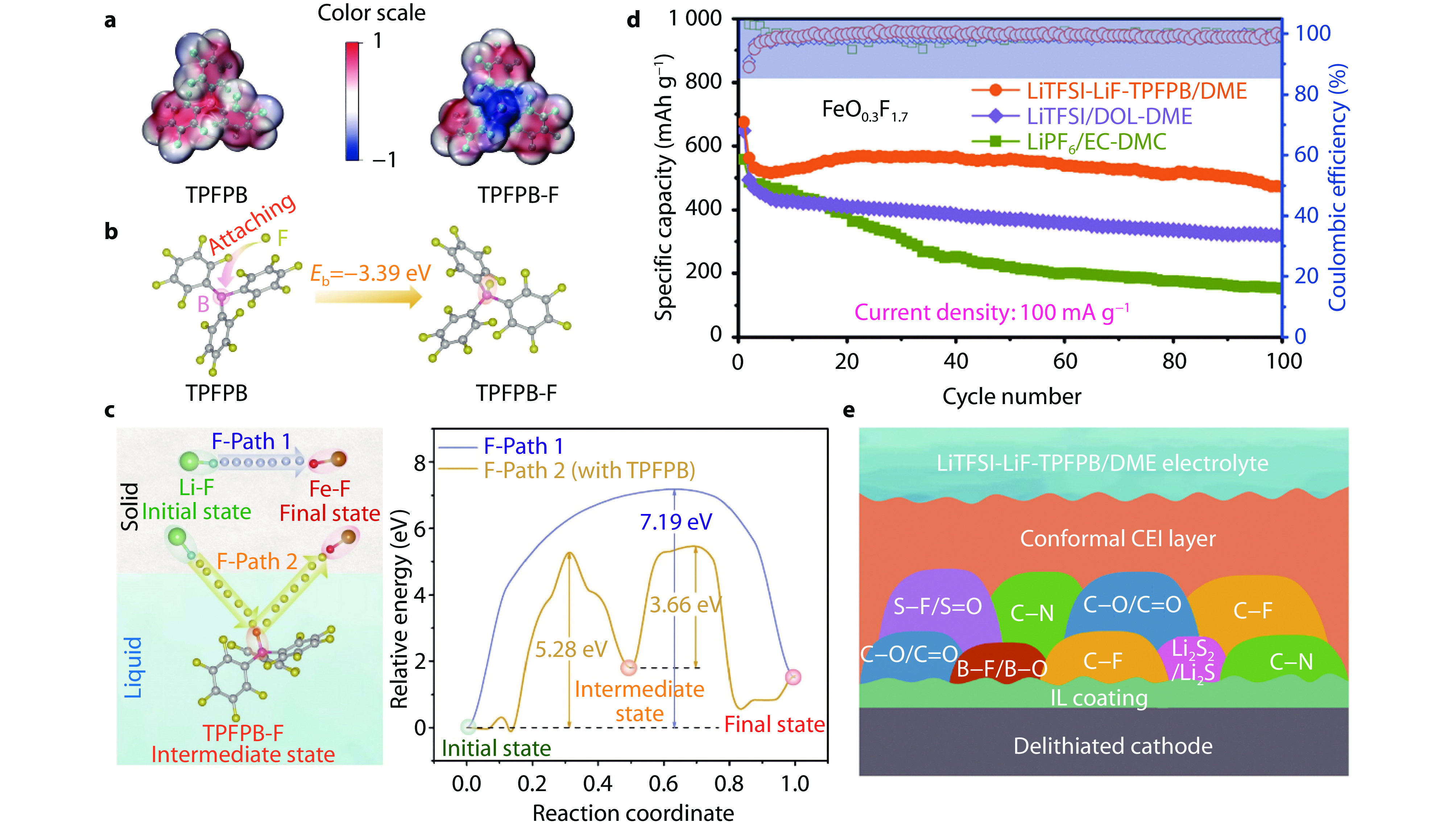
Figure 16.
Iron-based fluoride synthesized by FeF3.3H2O heat treatment and its coupling with LiTFSI-LiF-TPFPB/DME electrolyte. a Molecular structures and electrostatic potential analysis for TPFPB and TPFPB-F complex. b Binding energy of F atom towards central B element in TPFPB from DFT calculation. c Potential F-transport pathways and corresponding energy barriers for F transformation from Li-F to Fe-F with and without the assistance of TPFPB. d Cycling performance of FeO0.3F1.7 in different electrolytes. e Schematic illustration of CEI layer derived from LiTFSI-LiF-TPFPB/DME electrolyte after several cycles.[45] Copyright 2021, American Association for the Advancement of Science.

 Runyuan Cao received his Bachelor degree in Nanjing University of Science and Technology in 2022. He is currently a Ph.D. candidate in Shanghai Institute of Ceramics, Chinese Academy of Sciences under the supervision of Prof. Chilin Li. His major interest focuses on the research and development of high-capacity/long-cycling stability cathode materials for lithium-ion batteries.
Runyuan Cao received his Bachelor degree in Nanjing University of Science and Technology in 2022. He is currently a Ph.D. candidate in Shanghai Institute of Ceramics, Chinese Academy of Sciences under the supervision of Prof. Chilin Li. His major interest focuses on the research and development of high-capacity/long-cycling stability cathode materials for lithium-ion batteries.  Yifan Yu received his Bachelor degree in College of Materials Science and Engineering at Taiyuan University of Technology. He obtained his Ph.D. degree in Shanghai Institute of Ceramics, Chinese Academy of Sciences under the supervision of Prof. Chilin Li. His major interest focuses on the development of novel cathodes and electrolytes for rechargeable fluoride based batteries.
Yifan Yu received his Bachelor degree in College of Materials Science and Engineering at Taiyuan University of Technology. He obtained his Ph.D. degree in Shanghai Institute of Ceramics, Chinese Academy of Sciences under the supervision of Prof. Chilin Li. His major interest focuses on the development of novel cathodes and electrolytes for rechargeable fluoride based batteries.  Chilin Li received his Ph.D. degree in 2008 at Fudan University. He is now a professor in Shanghai Institute of Ceramics, Chinese Academy of Sciences (SICCAS). His research interest focuses on fluoride-based batteries, solid-state batteries, Li and Mg metal batteries, structure design and synthesis strategy of novel electrode and electrolyte materials, electrochemical mechanism, nanoionics.
Chilin Li received his Ph.D. degree in 2008 at Fudan University. He is now a professor in Shanghai Institute of Ceramics, Chinese Academy of Sciences (SICCAS). His research interest focuses on fluoride-based batteries, solid-state batteries, Li and Mg metal batteries, structure design and synthesis strategy of novel electrode and electrolyte materials, electrochemical mechanism, nanoionics. 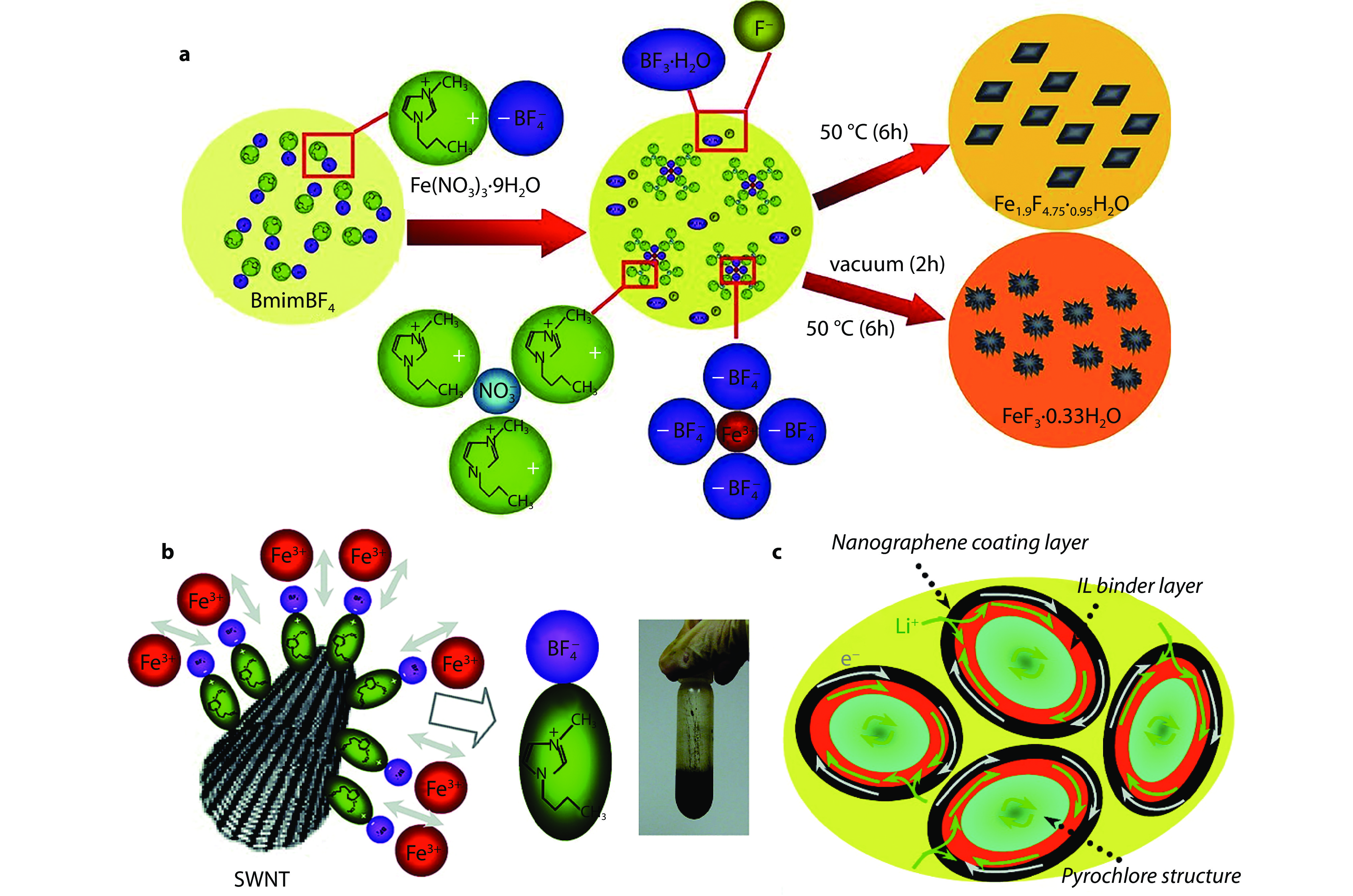

 DownLoad:
DownLoad: